Resource Centre
ASLM’s Resource Center is designed to help you keep up with the latest tool kits, regulations, guides and other information published by ASLM, partners, and global health regulatory bodies. Use the filters above to locate the information you need based on the ASLM project, resource type, or topic of interest. To find a specific resource, enter the resource name in the search bar. You may also use the search bar to enter key words related to the resources of your interest.
The SLIPTA Checklist V3, published by the World Health Organization (WHO), specifies requirements for quality and competency, aimed to develop and improve medical laboratory services to raise laboratory quality to established national and/or international standards. The elements of this checklist are based on ISO standard 15189:2022 (E) and, to a lesser extent, the Clinical &… Read More
In April 2023, ASLM’s Laboratory Directors Forum convened a high-level meeting on diagnostic integration and laboratory systems strengthening in Nairobi, Kenya. The meeting brought together 97 attendees, including 53 in-person and 44 virtual participants, consisting of laboratory directors, HIV and tuberculosis (TB) programme managers from 15 countries, global health experts, funders, and collaborating partners. This… Read More
The February 2023 LabCoP ECHO session is part 2 of The Revised ISO15189:2022 Standard series. It takes a deep dive into changes needed at country and facilities levels to align to and meet the requirements of the revised standard. The focus is on how to systematically handle the transition for both accredited and non-accredited laboratories…. Read More
This LabCoP ECHO session is the first in a four-part series dedicated to the revised ISO 15189:2022 version. In this session, we reintroduce key concepts of laboratory quality management systems (QMS) and cover some of the changes in the revised ISO 15189:2022, compared to the withdrawn ISO 15189:2012 version. In this session hosted by Beatrice… Read More
In LabCoP’s September ECHO session, ASLM and partners discussed optimising the TB/HIV diagnostic cascade through the implementation of the Clinic-Laboratory Interface Continuous Quality Improvement (CLICQ!) program. Optimal cascade performance requires that clinical and laboratory teams’ interface to ensure service continuity from identification of presumptive cases, specimen collection, transport and diagnostic testing, all the way through… Read More
This Guidance for Establishing a National Laboratory Quality Framework (NLQF), developed by Africa CDC and ASLM, has been prepared to guide and facilitate country-level efforts of strengthening national laboratory systems and networks. This document serves as a practical guide for countries during the development or updating of their national laboratory quality policies and strategic plans… Read More
In this In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we share the experience of Kenya in the development of a national quality manual and strategic plan for the animal health sector, supporting the establishment on a national quality infrastructure that supports and mandates quality management systems (QMS) implementation and external quality assurance (EQA)… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss our approach to establishing regional external quality assessment (EQA) providers and the achievements and lessons learnt from the implementation of a regional EQA programme for AMR. This AMR CoP and webinar are supported by the Fleming Fund’s EQA Grant for Africa (EQuAFRICA), together with… Read More
The Assay Verification Tool is designed to help laboratories implement new tests, making it easier to design, setup, and analyse data generated as part of a new test procedure verification. The tool provides planning assistance for assay verification experiments for PCR testing for CE-IVD assays. Data analysis of the performance specifications for assay verification is… Read More
On 10 March 2022, ASLM’s LabCoP convened a session on external quality assessment (EQA) for SARS-CoV-2 and other emerging viruses. EQA is a vital tool that provides objective evidence on the credibility of reporting test results, and labs are required to use it to monitor their performance. Conditions for the implementation of EQA vary, depending… Read More
On 28 October 2021 this Extended ECHO session focused on neglected tropical disease laboratories, their potential to act as effective reference labs with effective implementation of robust quality management systems (QMS) including external quality assessment (EQA) programs. Presentations were made by Dr Andreas Gilsdorf, Head, German Epidemic Preparedness Team SEEG), German Agency for International Cooperation… Read More
Laboratory diagnosis is an essential element of communicable disease surveillance, both for routine confirmation of infections and for the rapid identification of the cause of outbreaks and epidemics. An inaccurate diagnosis can have severe and expensive consequences such as inappropriate treatment or wastage of vaccines and test kits. Implementation of a strong quality management system… Read More
LabCoP’s January 2021 ECHO session covered PEPFAR’s Country Operational Plan 2021 and the laboratory systems strengthening priorities of the year. Dr George Alemnji, Senior Technical Advisor for PEPFAR Laboratory Services (OGAC), Washington DC, presented during the session. Please follow the links posted here to view the recorded video session on ASLM’s YouTube channel, and download… Read More
In December LabCoP conducted the inaugural session of the new LabCoP Extended series to introduce the Score-TB package, an integrated set of assessment tools for supporting the implementation of a QMS in laboratories performing tuberculosis tests. The Score-TB package was launched by Foundation for Innovative New Diagnostics (FIND), in collaboration with the Global Laboratory Initiative… Read More
In November 2020 the LabCoP stakeholders met online to convene the 2020 Face-to-Face virtual meeting. LabCoP’s 4th Annual Meeting aimed to assess the progress of LabCoP countries’ action plans and the outcome of ongoing interventions; discuss laboratory system strengthening across diseases through reviewing challenges and best practices in maintaining routine viral load (VL), early infant… Read More
On 12 November 2020, this COVID-19 ECHO session was convened to discuss the launch of the SARS-CoV-2 antigen rapid test training package developed by ASLM and Africa CDC, in partnership with CHAI, AFENET, AMREF, and Last Mile Health. The training package, available to all African Union Member States, aims to support the scale-up of SARS-CoV-2… Read More
ASLM in collaboration with the Africa Centres for Disease Control and Prevention, and in partnership with the Clinton Health Access Initiative, Amref and Last Mile Health present the Quality Assurance Framework for SARS-CoV-2 Antigen Rapid Testing for Diagnosis of COVID-19. This framework aims to provide general technical guidance to African Union Members States on the… Read More
LabCoP’s August ECHO session covered external quality assessment (EQA) proficiency testing coverage, results and sustainability from the perspective of a service provider. EQA provides a measure of performance of testing laboratories and helps identify deficiencies and opportunities for improvement across the entire testing cascade. In this session, we discuss integrating economic principles into EQA, to… Read More
On 6 August 2020, this COVID-19 ECHO session was convened to discuss laboratory data management considerations for COVID-19 response. Long turnaround times (TAT) for laboratory test results and poor data quality are chronic problems for many testing services. Timely and accurate testing services for COVID-19 are essential for monitoring the spread of the pandemic and… Read More
These updated guidelines (in English, French and Portuguese) for the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA), published by WHO, provide a framework for countries in the Africa Region in their efforts to strengthen medical laboratory services through fulfillment of the requirements of the ISO 15189 standard. The SLIPTA Guideline describes key elements of… Read More
In July of 2019, the World Health Organization and the African Society for Laboratory Medicine organized a meeting with countries and key stakeholders in diagnostics to discuss and find concrete ways to improve and increase access to integrated multiplex technologies and determine how they can be translated into public health policy that impacts global impact.
On 20 May 2020, this COVID-19 ECHO session was convened to discuss procurement and the supply chain for COVID-19 diagnostics. Dr Lara Vojnov, Diagnostics Advisor at the World Health Organization, Geneva, and Dr Yenew Kebede, Head of Laboratory Services at Africa CDC presented about the need to coordinate and collaborate test availability and access; current… Read More
On 14 May 2020, this COVID-19 ECHO session was convened to assist the African laboratory community in designing and implementing an effective COVID-19 quality assured testing program. Mr Patrick Mateta, Vice President, Global Health Partnerships, at Clinical and Laboratory Standards Institute (CLSI) presented about how to plan and prepare for COVID-19 testing, the selection and… Read More
On 8 May 2020, ASLM’s LabCoP hosted part 2 of an ECHO session about COVID-19 serology tests. Serological diagnostic testing has been proposed to be useful in combination with molecular testing, in confirming COVID-19 infection or exposure to the virus. Serology tests also have the potential to be delivered in rapid format and at the… Read More
On 30 April 202, ASLM’s LabCoP hosted the third session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott and Bio-Rad. Dr Danijela Lucic, Senior Scientific Affairs Manager, Global Infectious Disease, Abbott Molecular, and Dr Marcus Neusser, EMEA Product Manager, Gene Expression, Bio-Rad, Germany, and Mr Richie Petronis, Product Manager, Bio-Rad, USA,… Read More
On 29 April 2020, ASLM’s LabCoP hosted this ECHO session on strategies to implement fast turn-around laboratory testing for control of COVID-19, presented by Dr Trevor Peter, Senior Director, Diagnostic Services, Clinton Health Access Initiative. Nucleic acid testing is the WHO-recommended method for detecting SARS-CoV-2. The global scale-up of COVID-19 diagnostics will depend on use… Read More
On 24 April 2020, Professor Rosanna Peeling and colleagues from the International Diagnostics Centre at the London School of Hygiene and Tropical Medicine pulled together evidence for and against the original set of recommendations for use of serologic tests. Prof Peeling was joined by Dr Yenew Kebede from Africa CDC and Dr Amadou Sall from… Read More
Laboratories need to conduct verification or validation studies to confirm that new tests for COVID-19 diagnosis perform as intended. In this document co-authored by ASLM, FIND and the London School for Hygiene and Tropical Medicine, further key requirements for quality assurance are defined, including quality control and external quality assessment that laboratories should follow to… Read More
LabCoP’s April ECHO session featured presentations about maintaining HIV and TB testing in the context of COVID-19 from Dr George Alemnji, Senior Technical Advisor for Laboratory Services at the State Department Office of Global AIDS Coordinator (SGAC) and Health Diplomacy, US CDC; Dr Lara Vojnov, Diagnostics Advisor, WHO; and Dr Dennis Falzon, Medical Officer, World… Read More
On 16 April 2020, ASLM’s LabCoP hosted the second session in a series about manufacturers of COVID-19 molecular diagnostic tools. Dr Davide Manissero, Chief Medical Officer of Infection and Immune Diagnostics at QIAGEN, and Mr Riegardt Johnson, Field Application Scientist at Thermo Fisher Scientific presented about their available COVID-19 laboratory test kit(s), their status regarding… Read More
On 14 April 2020, ASLM’s LabCoP hosted the first session in a series about manufacturers of COVID-19 molecular diagnostics tools, including BD and Cepheid. Dr Charles Cooper, Global Vice President, Medical Affairs, Integrated Diagnostic Solutions at BD, and Ms Rujeko Tsomondo, Regional Application Lead at Cepheid presented about their available COVID-19 laboratory test kit(s), their… Read More
On 8 April 2020, ASLM and LabCoP convened a special ECHO session on lessons the African continent can learn from the Korean experience in controlling COVID-19 Dr Seon Kui Lee, Director, Division of Risk Assessment & International Cooperation at the Korea Centers for Disease Control and Prevention (KCDC), shared an overview of the KCDC and… Read More
On 30 March 2020, ASLM and LabCoP convened a special ECHO session on the latest available technology to diagnose COVID-19, and recommendations for clinical care and surveillance. Dr Cassandra Kelly-Cirino, from the Foundation for Innovative New Diagnostics (FIND), and Prof Rosanna Peeling, from the London School of Hygiene & Tropical Medicine, gave an overview of… Read More
On 25 March 2020, ASLM and LabCoP convened an ECHO session on troubleshooting common challenges associated with establishing diagnostic testing for SARS-CoV-2 (the causative agent of COVID-19). Dr Jinal Bhiman, Director of the National Influenza Centre at the NICD, South Africa, presented practical solutions to address the most common challenges encountered by laboratory staff recently… Read More
Pre-analytical challenges threaten the quality of viral load (VL) testing. This study determined the impact of delayed testing and warmer storage conditions on HIV RNA stability in diagnostic samples. Viral load in samples stored for up to a week reliably differentiated between patients with antiretroviral therapy (ART) suppression and ART failure in the majority of… Read More
Plasma preparation tubes (PPT) can simplify storage, transport, and preparation of plasma used for viral load (VL) testing. This systematic review evaluated the accuracy of PPTs for HIV VL testing. The results showed that following proper sample handling techniques helps provide accurate results. The authors thus recommended PPTs as a high-quality alternative specimen type for… Read More
This systematic review and meta-analysis summarizes evidence that shows that task shifting of testing using point-of-care technologies from laboratory professionals to non-laboratory staff was comparable to laboratory professionals operating the same technology in the laboratory. Task shifting of clinical tasks to lower cadres of health care workers and lay counselors has been successful in scaling… Read More
This paper by Katherine Lamp et al, 2019, outlines an analysis of point-of-care (POC) CD4 invalid result rates across five countries. The authors found that the rate of invalid results was consistent across all types of health facilities, and the invalid rates were inversely correlated to operator usage, with high-volume operators. They concluded that POC… Read More
In this session, colleagues from Kenya shared findings from the assessment of waste handling practices in their viral load (VL) and Early infant diagnosis (EID) laboratories. They discussed contextual issues in regard to VL & EID laboratory testing capacity and networks, laboratory self-assessment using a VL & EID customized assessment checklist, findings/results, lessons learnt, challenges… Read More
To reach the third 90 of the UNAIDS 90–90–90 targets, country programmes must delve into their data and understand how they represent the quality of viral load (VL) testing services. This guide, published by WHO, presents key considerations and examples of tools (provided in the annexes) to assist countries in developing a national VL M&E… Read More
The African Society for Laboratory Medicine (ASLM) has been designated by WHO-AFRO in October 2012 to coordinate and lead the implementation of the Stepwise Quality Laboratory Improvement Process towards Accreditation (SLIPTA) in Africa. ASLM has then developed a standardized SLIPTA auditor training curricula and trained the training at a country and regional levels. The training… Read More
The November 2019 Waste Management training session focused on the perspectives of Abbott Laboratories’ Best Practices for Waste Handling. Delfin Rubin, Global Product Manager of HIV Care for Infectious Disease Emerging Markets at Abbott Rapid Diagnostics, and Ami Soni, EHS Lead for Abbott Molecular describe the constituents of their HIV rapid point-of-care diagnostics, such as… Read More
In October 2019 the LabCoP stakeholders met in Addis Ababa, Ethiopia to convene the 2019 Face-to-Face meeting. It was co-convened by ASLM and the World Health Organization to enable countries to objectively assess their progress towards the improvement of the viral load testing (VLT) cascade, consolidate the implementation of interventions, enhance collaboration with civil society… Read More
Diagnostic network optimization is an exercise that aims to redesign the diagnostic network set-up in order to increase access, maximize impact, and generate efficiencies. It aligns testing demand and capacity in the most cost-effective way by defining the optimal instruments mix, identifying the most appropriate locations where instruments should be placed, and designing the referral… Read More
WHO has produced a biosafety video series entitled, “Good Microbiological Practice and Procedures (GMPP)”, which is central to the WHO Laboratory Biosafety Manual (LBM4) being revised and finalised. These seven training videos will help enhance the safety of any health laboratory, including those in resource-limited settings.
With limited funding for global health, identifying practical, cost and time-saving solutions, while also ensuring quality of care is increasingly important. One approach to increasing access to point-of-care (POC) testing is integrated testing (a term often used interchangeably with “multi-disease testing”), which tests for different conditions or diseases using the same diagnostic platform. This brief… Read More
Increased access to antiretroviral therapy (ART) and treatment monitoring for pregnant and breastfeeding women living with HIV is a priority for promoting health during the pregnancy and post-partum periods, and to minimize the risk of vertical transmission of HIV to infants. Point-of-care viral load (POC VL), which allows testing to be conducted at or near… Read More
Scaling up viral load (VL) testing to reach the third 95 of the UNAIDS 95-95-95 targets by 2030 requires a holistic approach, addressing each step of the VL testing continuum from the demand for the test for all eligible patients to the utilization of test results. Bottlenecks and inefficiencies along the VL testing cascade as… Read More
In this session we focused on the issues of policy, legal and regulatory frameworks for waste management, using an example from South Africa. This session underscored the fact one of the common noted challenges in many settings was that there were and still are limited or no existing national policies or guidelines on dealing with… Read More
This HIV viral load (VL) testing cascade self-assessment scorecard and user guide is used by the LabCoP country teams to identify critical gaps in their laboratory systems and assess the status of the different thematic areas of the VL cascade and their testing services to help identify opportunities for improvement. It was designed by the… Read More
This Waste Management session covered updates to the checklist for assessments of viral load (VL) and early infant diagnosis (EID) testing laboratories and associated healthcare facilities. David Bressler, MS, CBSP (SM) NRM of the International Laboratory Branch, CDC, Division of Global HIV and TB presented to the audience about the checklist/tool and its ability to… Read More
Integrating testing using multiplex technologies at the appropriate level of care can lead to more efficient and cost-effective testing services and can help to simplify and streamline other systems, such as specimen referral, human resources, and quality assurance. Integration should be a priority for both those countries with currently operational multi-disease testing devices and those… Read More
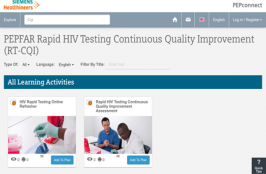
This Rapid Testing Continuous Quality Improvement (RTCQI) training video and competency assessment tool is available on a free virtual education platform, PEPconnect to assist ministries of health, health-care providers, and stakeholders in planning, implementing and sustaining quality assurance for point-of-care testing. Critical for the first of UNAIDS’ 90-90-90 targets, this platform is developed by the… Read More
The Stronger Together public-private partnership between Siemens Healthineers and PEPFAR is institutionalising quality improvement via a free e-platform, PEPconnect, through this library of 48 videos (with a total viewing time of 14.5 hours) that capture the lectures from the 9-day classroom-based Quality Control and Method Validation course. The course is designed to enable the participants… Read More
Early infant diagnosis (EID) is critical for timely initiation of antiretroviral treatment (ART) in HIV-infected children who are at high risk of mortality. In recognition of the immense benefits of dried blood spot (DBS) as a means of increasing the access to EID, the World Health Organization (WHO), African Society for Laboratory Medicine, Centers for… Read More
This guide, published by The Laboratory African Regional Collaborative (LARC) project, provides continuous quality improvement methodologies and tools. LARC supports clinicians, laboratorians and data record units to work together and improve the proportion of viral load test results put in patients’ files. LARC is a useful tool that can be used by countries to address… Read More
In the May 2019 session, Dr Katrina Sleeman, Associate Service Fellow in the Viral Load/Early Infant Diagnosis Team at the CDC ILB introduced a draft of a viral load WM checklist tool that can be used as baseline audit of select country VL labs. The tool aims to assist in creating awareness of best practices… Read More
In collaboration with ASLM and the LabCoP community, the CDC International Laboratory Branch (ILB) presented an overview of the World Health Organization (WHO) Publication: ‘Safe Management of Wastes from Health-Care Activities’ during the second ECHO session dedicated to waste management. This document, developed by WHO in 2017, highlights the key aspects of safe healthcare waste… Read More
This document, developed by the World Health Organization (WHO) in 2017, highlights the key aspects of safe healthcare waste management in order to guide policymakers, practitioners and facility managers to improve such services in healthcare facilities. It is based on the comprehensive WHO handbook ‘Safe Management of Wastes from Health-Care Activities’ (WHO, 2014), and also… Read More
The waste produced in the course of healthcare activities, from contaminated needles to radioactive isotopes, can cause infection and injury. Inadequate waste management is likely to have serious public health consequences and deleterious effects on the environment. The resources linked here and this downloadable handbook published by the World Health Organization (2014), provide comprehensive guidance… Read More
This four-step overview of Laboratory African Regional Collaborative (LARC) was presented by Patricia Riley from the International Laboratory Branch of the US Centers for Disease Control and Prevention. LARC can be used by countries to address inefficiencies at the laboratory–clinical interface at clinic level, and achieve the 3rd ‘90’ of the UNAIDS 90-90-90 HIV targets…. Read More