Resource Centre
ASLM’s Resource Center is designed to help you keep up with the latest tool kits, regulations, guides and other information published by ASLM, partners, and global health regulatory bodies. Use the filters above to locate the information you need based on the ASLM project, resource type, or topic of interest. To find a specific resource, enter the resource name in the search bar. You may also use the search bar to enter key words related to the resources of your interest.
The Laboratory System’s Strengthening Community of Practice (LabCoP) 7th Annual Meeting report presents key summaries, proposed actions and recommendations from the 2023 annual meeting. Held in Cape Town, South Africa on 11 December 2023, on the sidelines of the ASLM2023 conference, the meeting brought together 22 country teams, funding agencies, ASLM collaborating partners and the… Read More
ASLM’s LabCoP team is excited to release the latest recipe in the LabCoP Cookbook of Best Practices: Expanding Access to World Health Organization-Recommended Rapid Molecular Diagnostic Tools For Tuberculosis – Truenat as a Case Scenario.
In April 2023, ASLM’s Laboratory Directors Forum convened a high-level meeting on diagnostic integration and laboratory systems strengthening in Nairobi, Kenya. The meeting brought together 97 attendees, including 53 in-person and 44 virtual participants, consisting of laboratory directors, HIV and tuberculosis (TB) programme managers from 15 countries, global health experts, funders, and collaborating partners. This… Read More
The October 2023 LabCoP Diagnostic Network Optimization (DNO) Sub-CoP EHO Session was help in conjunction with the Kenya Ministry of Health and partners on integrated DNO. The session provided an overview of the integrated DNO conducted in Kenya to improve access to testing for tuberculosis (TB), HIV and human papillomavirus (HPV). Presentations were made by… Read More
The August LabCoP ECHO session, was jointly organized by ASLM and Global Laboratory Initiative (GLI), one of the 7 working groups of the Stop TB Partnership. This session was convened to review the World Health Organization’s Standard on Universal Access to Rapid Tuberculosis Diagnostics. The WHO End TB Strategy calls for all notified TB cases… Read More
The May 2023 LabCoP ECHO session jointly organised by the African Society for Laboratory Medicine and the Global Laboratory Initiative (GLI) featured the Tuberculosis Diagnostic Network Assessment (TB-DNA) tool. TB-DNA is a country-driven process that assesses the functionality of the national tuberculosis (TB) diagnostic network and system to determine its capacity to meet the country’s… Read More
Safe disposal of laboratory waste is a critical component of good laboratory practice. Molecular diagnostic test services, including HIV viral load (VL) and coronavirus disease 2019 (COVID-19) testing, have the potential to generate guanidinium thiocyanate (GTC), a potentially toxic compound, which requires specialised treatment and disposal. There are growing waste management challenges in many laboratories… Read More
This WHO publication sets benchmarks to achieve universal access to WHO-recommended rapid diagnostics (WRDs), increase bacteriologically confirmed tuberculosis and drug resistance detection, and reduce the time to diagnosis. WHO-recommended rapid diagnostics are highly accurate, cost-effective, reduce the time to treatment initiation, and impact patient-important outcomes. The WHO Standard comprises twelve benchmarks to be computed by… Read More
On 4 May, ASLM’s LabCoP, in collaboration with EDCTP and TB-CAPT, convened an Extended ECHO session that focused on exploring advancements in the lipoarabinomannan (LAM) diagnostic development pipeline, approaches for clinical adoption in field settings, and strategies for quality assessment. The information in this session helps address the urgent need for TB tools and diagnostic… Read More
This LabCoP Diagnostic Network Optimisation (DNO) Sub-CoP ECHO session in co-convened with the Foundation for Innovative New Diagnostics (FIND), focuses on providing an overview of the DNO analysis in Zambia for TB/HIV testing integration, including objectives, methodology and key findings. It further explores how findings from the diagnostic network optimization analysis informed Zambia Ministry of… Read More
The March 2023 LabCoP ECHO session organised by ASLM and the Global Laboratory Initiative (GLI) focused on the GLI Planning and Budgeting Tool for tuberculosis and drug resistant TB testing, and the Stop TB Partnership Global Drug Facility (GDF) diagnostics product catalogue. These planning and budgeting tools are semi-automated, editable, Excel-based calculation tools for estimating… Read More
The Laboratory System’s Strengthening Community of Practice (LabCoP) 6th Annual Meeting report presents key summaries, proposed actions and recommendations from the 2022 annual meeting. Held in Cape Town, South Africa from 11-13 October 2022, this hybrid in-person and virtual meeting brought together 19 country teams, funding agencies, ASLM collaborating partners and the LabCoP Management Team…. Read More
In LabCoP’s September ECHO session, ASLM and partners discussed optimising the TB/HIV diagnostic cascade through the implementation of the Clinic-Laboratory Interface Continuous Quality Improvement (CLICQ!) program. Optimal cascade performance requires that clinical and laboratory teams’ interface to ensure service continuity from identification of presumptive cases, specimen collection, transport and diagnostic testing, all the way through… Read More
This LabCoP Diagnostic Network Optimisation (DNO) Sub-CoP ECHO session focuses on promoting data-driven investments for effective laboratory network optimisation. In this session, the Global Fund, provides an overview of the GF funding cycle, priorities for involvement of Lab Directorates in preparing funding request, funding for diagnostic network optimization and how to plan the DNO lifecycle… Read More
On 31 May 2022, ASLM, in collaboration with the Stop TB Partnership Global Laboratory Initiative (GLI), EDCTP, and TB-CAPT project, convened a session on guidance in selecting patient-cantered, setting-specific solutions for tuberculosis (TB) detection. This new WHO/ Global Laboratory Initiative manual recommends that all patients with presumptive TB receive an initial, molecular WHO-recommended diagnostic (mWRD)… Read More
On 14 April 2022 ASLM’s LabCoP convened an Extended ECHO session about addressing diagnostic gaps in the tuberculosis (TB) care cascade, which featured discussion about the MTB/XDR assays and considerations for their implementation. Presentations were made by Dr. Kogieleum Naidoo, Head of the HIV and Tuberculosis Treatment Program at the Centre for AIDS Program of… Read More
On 7 April, ASLM’s LabCoP convened an Extended ECHO session about the role of diagnostic network optimization to achieve 95-90-0 for mycobacterium tuberculosis (MTB) disease elimination while scaling up testing. This webinar provided perspectives from Roche Diagnostics on the role of network optimisation to achieve 95-90-0 for MTB disease elimination; the deployment of some of… Read More
On 27 Jan 2022, this extended ECHO session was convened to review the experiences from Tanzania’s first Tuberculosis Diagnostic Network Assessment. The aim of this assessment was to holistically understand the TB diagnostic network, identify key strengths and weaknesses and provide evidence-based recommendations to improve the network in Tanzania. The presentation was made by Samwel… Read More
The World Health Organization’s 2021 Global Tuberculosis Report presents key facts, global findings, commitments, strategies and targets on tuberculosis (TB). The report demonstrates an overall drop in performance and reversal of gains in both diagnosis and treatment of TB achieved over the years leading to the SARS-CoV-2 pandemic in most of the six WHO regions…. Read More
On 14 October 2021 this Extended ECHO session was convened to discuss the basics of latent tuberculosis infection (LTBI), the critical importance of screening for LTBI in addition to active TB case finding. Experts discussed gaps in the current TB care cascade, considered various options for LTBI screening and highlighted the benefits of the test-and-treat… Read More
Essential diagnostic tests satisfy priority healthcare needs of a population and are selected based on disease prevalence, public health relevance, evidence of utility and comparative cost-effectiveness. Whereas essential testing services are such a critical piece in disease diagnosis, treatment monitoring, research and ongoing surveillance; pandemic containment measures, including repurposing testing capacity to the response, quarantine,… Read More
On 15 July 2021 this Extended ECHO session was convened to discuss the WHO`s End TB Strategy that requires universal drug susceptibility testing and treatment of all tuberculosis case. Fewer than desired drug-resistant tuberculosis cases receive appropriate treatment due to either limited access to or sophisticated laboratory infrastructure needs. This webinar provided details on the… Read More
On 30 June 2021 this Extended ECHO session was convened to discuss the implementation of the Lateral-Flow Urine Lipoarabinomannan (LF-LAM) assay for the detection of active tuberculosis (TB) in people living with HIV. The session provided an overview of current World Health Organisation (WHO) recommendations, practical considerations to increase uptake, LF-LAM inclusion within the package… Read More
On 29 April, 2021 this COVID-19 ECHO session was convened to discuss tuberculosis (TB) and the COVID-19 pandemic. This panel discussion session was moderated by Rinuka Garde, Senior Advisor at the Clinton Health Access Initiative, and included three renowned experts in the field of TB and general lung health, including: Lucica Ditu, Executive Director Stop… Read More
This presentation by the Clinton Health Access Initiative outlines the steps of an integration implementation framework for tuberculosis (TB) and HIV diagnostics, and related diseases, top tips and caveats (what could go wrong and lessons learned) from pilot countries on each step and area of implementation. It provides a deeper understanding of activities required for… Read More
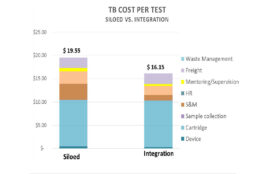
This presentation by the Clinton Health Access Initiative outlines the need for point-of-care (POC) testing, how integrated testing improves access to POC, the benefits of integration, and considerations and implementation framework for integrated testing.
The GX Capacity Utilisation Analysis Tool is an Excel-based tool to assess the capacity utilisation of GeneXpert platforms for integrating tuberculosis (TB), HIV, HCV and HPV testing. It has been used to identify platforms running with full-capacity and with un-used capacity to inform integration decisions in Cameroon, Ethiopia, Indonesia, Malawi, Nigeria, Tanzania, Uganda, Zambia, and… Read More
The Integration Readiness Assessment Tool developed by Clinton Health Access Initiative and partners helps countries gather situation analysis information regarding an individual facility’s readiness to provide integrated testing for tuberculosis (TB), HIV and HPV. The assessment output of this MS Excel-based checklist allows for identification of sites where TB/HIV/HCV/HPV integration on the GeneXpert platform would… Read More
LabCoP’s January 2021 ECHO session covered PEPFAR’s Country Operational Plan 2021 and the laboratory systems strengthening priorities of the year. Dr George Alemnji, Senior Technical Advisor for PEPFAR Laboratory Services (OGAC), Washington DC, presented during the session. Please follow the links posted here to view the recorded video session on ASLM’s YouTube channel, and download… Read More
In December LabCoP conducted the inaugural session of the new LabCoP Extended series to introduce the Score-TB package, an integrated set of assessment tools for supporting the implementation of a QMS in laboratories performing tuberculosis tests. The Score-TB package was launched by Foundation for Innovative New Diagnostics (FIND), in collaboration with the Global Laboratory Initiative… Read More
In November 2020 the LabCoP stakeholders met online to convene the 2020 Face-to-Face virtual meeting. LabCoP’s 4th Annual Meeting aimed to assess the progress of LabCoP countries’ action plans and the outcome of ongoing interventions; discuss laboratory system strengthening across diseases through reviewing challenges and best practices in maintaining routine viral load (VL), early infant… Read More
In July of 2019, the World Health Organization and the African Society for Laboratory Medicine organized a meeting with countries and key stakeholders in diagnostics to discuss and find concrete ways to improve and increase access to integrated multiplex technologies and determine how they can be translated into public health policy that impacts global impact.
Laboratories need to conduct verification or validation studies to confirm that new tests for COVID-19 diagnosis perform as intended. In this document co-authored by ASLM, FIND and the London School for Hygiene and Tropical Medicine, further key requirements for quality assurance are defined, including quality control and external quality assessment that laboratories should follow to… Read More
LabCoP’s April ECHO session featured presentations about maintaining HIV and TB testing in the context of COVID-19 from Dr George Alemnji, Senior Technical Advisor for Laboratory Services at the State Department Office of Global AIDS Coordinator (SGAC) and Health Diplomacy, US CDC; Dr Lara Vojnov, Diagnostics Advisor, WHO; and Dr Dennis Falzon, Medical Officer, World… Read More
Mozambique provided this Excel-based tool, with its accompanying instructions, as a country example of a comprehensive diagnostic network optimisation or mapping tool at the July 2019 Global Diagnostics Integration meeting in Geneva. The government sought a tool that could be: subnational, consider multiple potential technologies as well as both laboratory-based and point-of-care, incorporate testing across… Read More
Developed by the Clinton Health Access Initiative (CHAI) for its country teams and ministries of health, this tool can be used to assess the financial benefits of integrating tuberculosis (TB), HIV, and HCV testing on the GeneXpert machine. The tool only covers cross-cutting costs (i.e. not disease-specific) regarding the use of GeneXpert, such as equipment,… Read More
These presentation slides are from the Women-Centered Diagnostics satellite session of the 2019 International Conference on AIDS and STIs in Africa (ICASA 2019) that was jointly organized by the African Society for Laboratory Medicine (ASLM), the Clinton Health Access Initiative (CHAI), the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), UNICEF and Unitaid. Much has been done… Read More
The purpose of this tool is to assist in identifying gaps and creating awareness of best practices for waste management processes in viral load (VL) and early infant diagnosis (EID) molecular testing laboratories (and associated healthcare facilities), in order to provide a starting point for assistance in waste mitigation strategies. This tool is for completion… Read More
In October 2019 the LabCoP stakeholders met in Addis Ababa, Ethiopia to convene the 2019 Face-to-Face meeting. It was co-convened by ASLM and the World Health Organization to enable countries to objectively assess their progress towards the improvement of the viral load testing (VLT) cascade, consolidate the implementation of interventions, enhance collaboration with civil society… Read More
Diagnostic network optimization is an exercise that aims to redesign the diagnostic network set-up in order to increase access, maximize impact, and generate efficiencies. It aligns testing demand and capacity in the most cost-effective way by defining the optimal instruments mix, identifying the most appropriate locations where instruments should be placed, and designing the referral… Read More
With limited funding for global health, identifying practical, cost and time-saving solutions, while also ensuring quality of care is increasingly important. One approach to increasing access to point-of-care (POC) testing is integrated testing (a term often used interchangeably with “multi-disease testing”), which tests for different conditions or diseases using the same diagnostic platform. This brief… Read More
This document, developed by the World Health Organization (WHO) in 2017, highlights the key aspects of safe healthcare waste management in order to guide policymakers, practitioners and facility managers to improve such services in healthcare facilities. It is based on the comprehensive WHO handbook ‘Safe Management of Wastes from Health-Care Activities’ (WHO, 2014), and also… Read More