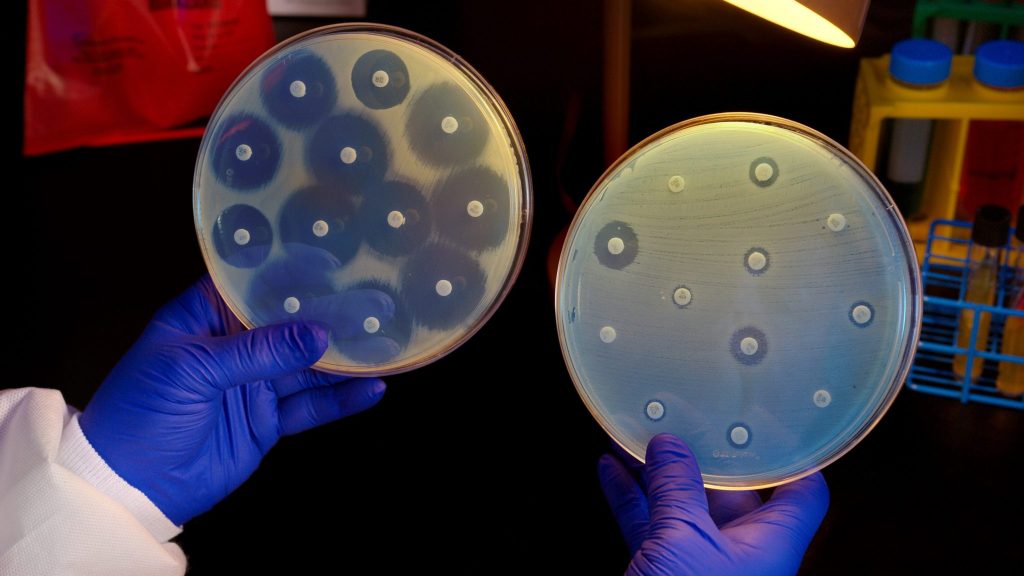
AMR CoP ECHO Session 12 July 2024 – The Rise of Resistance: How Bacteria Outsmart Antibiotics
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss the sophisticated and crafty methods bacteria use to resist antibiotics. The session presentation is del
July 2024 LabCoP Monthly ECHO Session – Setting up and Running a Sustainable National Equipment Maintenance and Calibration Center: Experience from Uganda
On 11 July 2024, ASLM’s LabCoP hosted a monthly ECHO session that focused on setting up and running a sustainable national equipment maintenance and calibration center and the ex
WHO’s Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) Checklist
The SLIPTA Checklist V3, published by the World Health Organization (WHO), specifies requirements for quality and competency, aimed to develop and improve medical laboratory servic
LabCoP Cookbook Recipe #8: Expanding Access to World Health Organization-Recommended Rapid Molecular Diagnostic Tools For Tuberculosis – Truenat as a Case Scenario
Tuberculosis (TB) remains a major public health challenge and a leading cause of death by an infectious disease. The World Health Organization (WHO) has set targets to reduce
The Role of HIV Viral Suppression in Improving Individual Health and Reducing Transmission: WHO Policy Brief
This World Health Organization policy brief explains the rationale behind the thresholds of HIV viral load suppression and provides additional information regarding implementation
Building Testing Capacity for Epidemic-Prone Diseases
Through its Africa Task Force for Novel Coronavirus (AFTCOR) Laboratory Technical Working Group, the Africa Centres for Disease Control and Prevention (Africa CDC) has developed gu
LabCoP Cookbook Recipe #7: Management of Guanidinium Thiocyanate Containing Waste From Testing Laboratories
Safe disposal of laboratory waste is a critical component of good laboratory practice. Molecular diagnostic test services, including HIV viral load (VL) and coronavirus disease 201
The WHO Standard: Universal Access to Rapid Tuberculosis Diagnostics
This WHO publication sets benchmarks to achieve universal access to WHO-recommended rapid diagnostics (WRDs), increase bacteriologically confirmed tuberculosis and drug resistance
February 2023 LabCoP Monthly ECHO Session: Adopting the WHO-Recommended Three-Test Algorithm for Improving the Quality of HIV Diagnosis
A second February 2023 LabCoP Monthly ECHO session is one of a two-part series dedicated to the new WHO recommended HIV test algorithm. In this first session, we focus on transitio
February 2023 LabCoP ECHO Session: The Revised ISO 15189:2022 Standard Part 2: Implications at the Country and Facility Levels
The February 2023 LabCoP ECHO session is part 2 of The Revised ISO15189:2022 Standard series. It takes a deep dive into changes needed at country and facilities levels to align to