Resource Centre
ASLM’s Resource Center is designed to help you keep up with the latest tool kits, regulations, guides and other information published by ASLM, partners, and global health regulatory bodies. Use the filters above to locate the information you need based on the ASLM project, resource type, or topic of interest. To find a specific resource, enter the resource name in the search bar. You may also use the search bar to enter key words related to the resources of your interest.
This policy paper, published by the Center for Global Development, qualitatively establishes the complex costs and benefits of strengthening laboratory capacity and systems within and across national borders. Costs are presented as direct and indirect, while benefits are presented at the individual, population, and health-system levels. Each cost and benefit grouping is further divided into… Read More
The study collected information from African countries about existing national guidance documents and the current process of decision making when selecting tier-specific in-vitro diagnostics (IVD). The lessons learnt represent useful insights on what works and what does not, that could be the basis for recommendations to supporting countries and stakeholders in developing and implementing a… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss the One Health approach to addressing antimicrobial resistance (AMR). This approach provides significant opportunities to explore broadly-existing microbial relationships between humans and animals. In this session, from the genomic point of view, the Institut Pasteur de Dakar (IPD), Senegal presents unique perspectives of AMR… Read More
On 8 December 2021 this special ECHO session was held to focus on the role of the Diagnostic Evidence Hub in accelerating uptake of diagnostic innovations. The Diagnostic Evidence Hub, launched in 2020 by the FASTER Project and hosted on the ASLM website, provides easy access to consolidated regulatory and performance data for test methods… Read More
This presentation by the Clinton Health Access Initiative outlines the steps of an integration implementation framework for tuberculosis (TB) and HIV diagnostics, and related diseases, top tips and caveats (what could go wrong and lessons learned) from pilot countries on each step and area of implementation. It provides a deeper understanding of activities required for… Read More
This presentation by the Clinton Health Access Initiative outlines the need for point-of-care (POC) testing, how integrated testing improves access to POC, the benefits of integration, and considerations and implementation framework for integrated testing.
The GX Capacity Utilisation Analysis Tool is an Excel-based tool to assess the capacity utilisation of GeneXpert platforms for integrating tuberculosis (TB), HIV, HCV and HPV testing. It has been used to identify platforms running with full-capacity and with un-used capacity to inform integration decisions in Cameroon, Ethiopia, Indonesia, Malawi, Nigeria, Tanzania, Uganda, Zambia, and… Read More
The Integration Readiness Assessment Tool developed by Clinton Health Access Initiative and partners helps countries gather situation analysis information regarding an individual facility’s readiness to provide integrated testing for tuberculosis (TB), HIV and HPV. The assessment output of this MS Excel-based checklist allows for identification of sites where TB/HIV/HCV/HPV integration on the GeneXpert platform would… Read More
In July of 2019, the World Health Organization and the African Society for Laboratory Medicine organized a meeting with countries and key stakeholders in diagnostics to discuss and find concrete ways to improve and increase access to integrated multiplex technologies and determine how they can be translated into public health policy that impacts global impact.
Mozambique provided this Excel-based tool, with its accompanying instructions, as a country example of a comprehensive diagnostic network optimisation or mapping tool at the July 2019 Global Diagnostics Integration meeting in Geneva. The government sought a tool that could be: subnational, consider multiple potential technologies as well as both laboratory-based and point-of-care, incorporate testing across… Read More
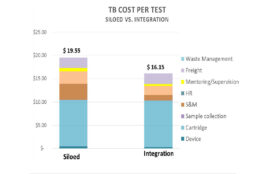
Developed by the Clinton Health Access Initiative (CHAI) for its country teams and ministries of health, this tool can be used to assess the financial benefits of integrating tuberculosis (TB), HIV, and HCV testing on the GeneXpert machine. The tool only covers cross-cutting costs (i.e. not disease-specific) regarding the use of GeneXpert, such as equipment,… Read More
With limited funding for global health, identifying practical, cost and time-saving solutions, while also ensuring quality of care is increasingly important. One approach to increasing access to point-of-care (POC) testing is integrated testing (a term often used interchangeably with “multi-disease testing”), which tests for different conditions or diseases using the same diagnostic platform. This brief… Read More
Integrating testing using multiplex technologies at the appropriate level of care can lead to more efficient and cost-effective testing services and can help to simplify and streamline other systems, such as specimen referral, human resources, and quality assurance. Integration should be a priority for both those countries with currently operational multi-disease testing devices and those… Read More