ECHO Sessions Summary: January to August 2023
The African Society for Laboratory Medicine (ASLM), in collaboration with partners, continued its successful series of information, knowledge and experience-sharing ECHO sessions through its flagship Laboratory Systems Strengthening Community of Practice (LabCoP) project. Below is an overview of the key sessions conducted between January 2023 and August 2023.
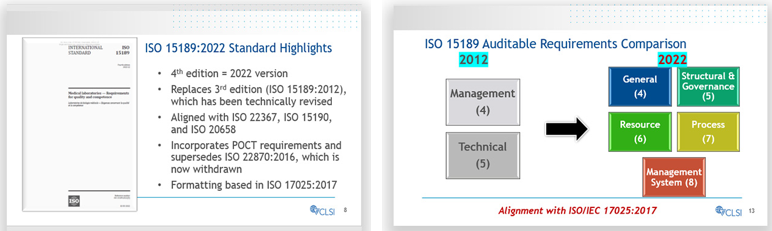
The Revised ISO 15189:2022 Series
Following the release of ISO 15189:2022 in December 2022, two sessions were delivered to highlight key changes in the new version compared to the previous one. The webinars both explained the implications of these changes and offered guidance for national, facility-level and individual laboratories during the provided three-year transition period. Watch the ISO 15189:2022 ECHO sessions here.
Diagnostic Network Optimisation SubCoP
In collaboration with the Foundation for Innovative New Diagnostics (FIND), ASLM hosted the second Diagnostic Network Optimisation (DNO) Sub-Community of Practice (SubCoP) session in April 2023. The session focused on DNO analysis, drawing on experience from Zambia with integrating HIV and tuberculosis (TB) testing. It explored how findings from DNO analysis informed ministry of health strategies to enhance HIV and TB testing accessibility for vulnerable populations. Watch the DNO SubCoP ECHO sessions here.
Waste Management SubCoP
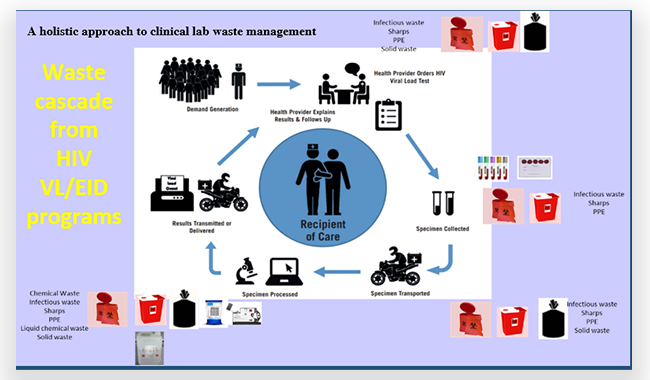
LabCoP’s Waste Management Sub-Community of Practice conducted its second phase of ECHO sessions in a three-part series from February 2023 to July 2023. The first session concentrated on moving from awareness creation to action, including how to rally stakeholders to identify and implement safe, practical, and sustainable methods and technologies for healthcare waste disposal. The subsequent sessions, held in June and July, were the result of a collaboration with the United Kingdom’s Health Security Agency and delved into fundamental principles, procedures, precautions and mitigation measures when handling chemical hazards. Additionally, these sessions covered introductory-level information on chemical detection, common surveillance methods for identification of potential public health concerns related to chemicals and the role of poison information centres in supporting detection and response to chemical events. Watch the Waste Management SubCoP ECHO sessions here.
The Diagnostic Resolution of the 76th World Health Assembly
In June 2023, a special session was convened to discuss the historic World Health Assembly resolution on strengthening diagnostics capacity. This ground-breaking resolution was initiated by Eswatini and co-sponsored by all World Health Organization (WHO) Regional Office for Africa member states, Indonesia, Bangladesh, Germany, France, Canada and others. The session outlined 15 diagnostics-strengthening steps for member states, as well as an additional 16 steps for the WHO Director General. Watch the special ECHO session here.
Tools, Global Guidelines, Standards and Country-Level Experiences
In May 2023, ASLM conducted five sessions covering critical tools for HIV and TB diagnostics, planning and budgeting, and testing algorithms. This included a session about the semi-automated tool developed by Global Laboratory Initiative and partners for estimating quantities and costs of diagnostic products and laboratory supplies. Watch the session here. Another session introduced the TB Diagnostic Network Assessment (TB-DNA) Tool, designed to assess the functionality and capacity of national TB diagnostic networks. Watch the session here. In February, LabCoP held a session on guidance for deploying rapid screening for malaria to low-transmission zones to accelerate malaria elimination. Watch the session here. In March, two guidelines and standards were presented, including the WHO-recommended three-test algorithm for improving quality of HIV diagnosis, which emphasises the need for three reactive tests to provide a positive HIV diagnosis. Watch the session here. Lastly, the August session introduced the WHO standards for universal access to rapid TB diagnostics and highlighted 12 benchmarks across four steps of the diagnostic cascade that countries should focus on to monitor and use the results to improve program performance. Watch the session here.

