ECHO sessions between April and June focused heavily on tuberculosis (TB) diagnostics, including a series commemorating the World TB Day. The sessions also highlighted technologies that are simplifying processes in activities such as diagnostic assay verification, the Rapid Test Continuous Quality Improvement (RTCQI) initiative, human papillomavirus screening, and the implementation of electronic return of results to health workers and recipients of care. Finally, one session focused on the role of lay workers to advance diagnostics at the community level.
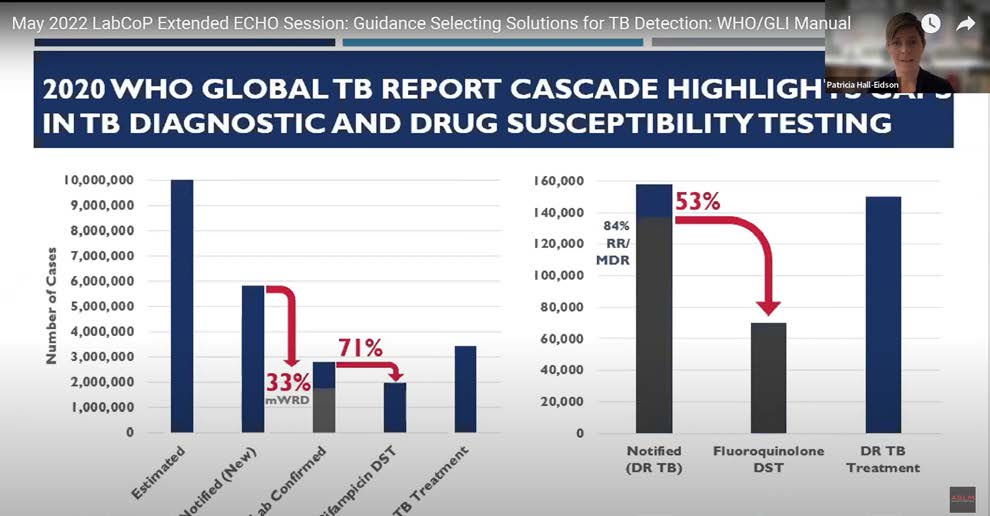
Slide from Patricia Hall-Eidson’s (ILB US CDC) presentation shows continued gaps in patient access to essential TB testing services
The TB testing landscape is rapidly expanding, providing more options for patient-centred testing strategies. The recent Manual for Selection of Molecular WHO-recommended Rapid Diagnostic Tests (mWRD) for Detection of TB and Drug-resistant TB suggests that TB test selection and associated network analyses should be guided by setting specific patient and programmatic needs. The guide advises on considerations for key elements such as test performance costs, procurement and supply chain, and infrastructure and human resource requirements for selecting mWRD for specific settings. View the session here.
The TB series also highlighted the role of diagnostic network optimisation to achieve global targets[1] for Mycobacterium tuberculosis (MTB) disease elimination, including new testing options to strategically complement the existing near-patient capabilities by considering the geographic area, the volume of testing required, and multi-disease testing needs to improve programmatic results and facilitate TB eradication. View the session here.
Evaluation studies in South Africa on how the Xpert MTB/XDR assay addresses gaps in the current drug-resistant tuberculosis (DR-TB) diagnostic pathway demonstrated it to be a suitable assay for detecting resistance to isoniazid, fluoroquinolones and second-line injectable drugs. To address existing challenges such as low rates of follow-on drug-susceptibility testing and treatment initiation, novel approaches to expand testing and ensure appropriate treatment initiation for undiagnosed and untreated patients are still required. View the session here.
With the introduction of new diagnostics, e.g., TB diagnostics as exemplified above, expertise in conducting and performing the required analyses for assay verification is scarce, even at the reference laboratory level in many settings in Africa. To address this critical gap in quality management systems, a public-private partnership between Roche and the United States Centers for Disease Control and Prevention, in collaboration with ASLM, developed the Assay Verification Tool, now available free on ASLM’s Resources Centre. This tool simplifies the analyses for verifications, validations, and other laboratory evaluations. Learn more about the tool in the recorded session here.
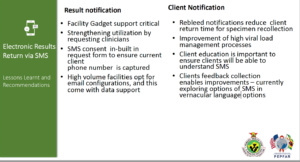
Fig 1: Some lessons from the implementation of SMS electronic results delivery in Zimbabwe
While much effort has been made on patient diagnostics, the utilisation of test results remains a significant gap in many countries. For example, progressive LabCoP annual country self-assessment reports continue to show the inability to track patients with high viral load and to determine if these patients eventually receive enhanced adherence counselling, switch to a second-line ART regimen, and finally achieve viral suppression. Implementation of short message service (SMS) for electronic results return directly to health facilities and/or to patients has shown to be promising in some countries. The June ECHO session highlighted the positive outcome and key lessons learned from a pilot program in Zimbabwe in which there was a near 10-fold reduction in turn-around time to result delivery to patients (see Fig 1). Watch more of the session here.
To address the shortfall in the laboratory workforce, lay workers have been engaged to support testing services, especially for diagnostics at decentralised health facilities, where patients initially seek healthcare support. However, this practice has not been adopted to scale, primarily due to barriers such as a lack of explicit national policies promoting task shifting and integration of lay cadres into human resource structures in national programs. In addition, in some cases, laboratory professional regulatory councils, associations, and laboratory technicians have resisted the endorsement of task shifting for specific point-of-care tests. Learn more by watching the session here.
[1] World Health Organization’s End TB Strategy (2016–2035) and the Sustainable Development Goals (2016–2030).
