Resource Centre
COVID-19 ECHO Session #28: Manufacturers of COVID-19 Rapid Diagnostics Tests: Abbott Panbio
October 22, 2020
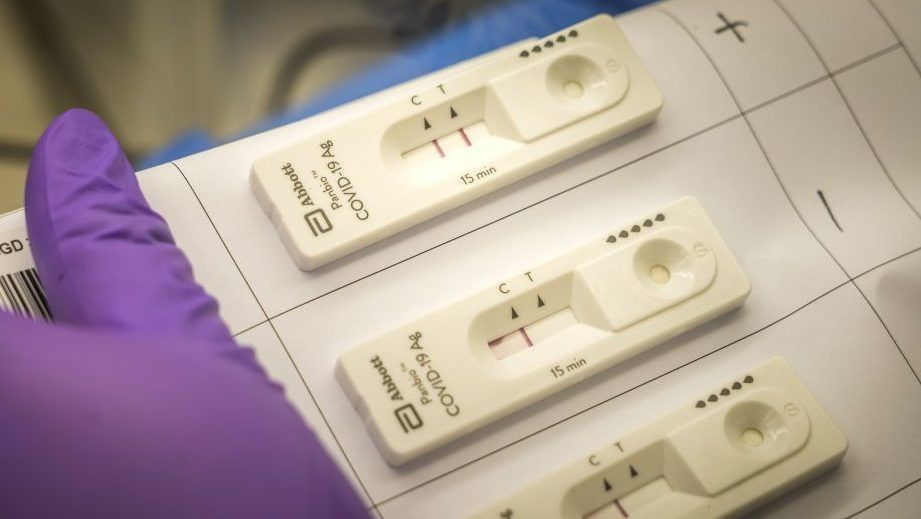
On 21 October, 2020 ASLM’s LabCoP hosted the ninth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott’s Panbio Ag RDT, an in-vitro diagnostic test for the qualitative detection of SAR-CoV-2 antigen. In this session, Dr Kuku Appiah, Director, Medical & Scientific Affairs, Africa, Abbott Rapid Diagnostics discuss the Abbott Panbio COVID-19 rapid antigen product overview; the performance, run time, and other technical considerations; clinical results; requirements and consumables for testing; the detailed status regarding regulatory approval for Emergency Use Listing (EUL); and suggested in-country protocols. She also discusses the Sympheos system – a suite for rapid testing and software tools for COVID-19 decentralised testing. It provides a real-time reporting and visualisation of the COVID-19 hotspots, outbreaks, and epidemiological statics that help the MoH Program Managers to efficiently deploy and manage scarce resources. Please follow the links posted here to view the recorded video session on ASLM’s YouTube channel and download the presentation slides from the session. Please note that ASLM does not endorse specific manufacturers over others. This session is for informational purposes only.

