Point-of-Care Testing: Why is it important?
Imagine yourself in a city at the centre of one of the world’s deadliest disease outbreaks. You watch as your friends and relatives are struck down by an aggressive illness that kills roughly 50-90% of those affected. One evening, you go to bed tired and achy, awakening the next morning with fever and headache. You do the right thing and call the emergency number. You are told to head to the nearest healthcare facility, a holding centre far from your neighbourhood. You are greeted at the holding centre by security personnel wearing face masks, face shields and gloves. From a distance, they tell you to wash your hands in bleach solution and then point you through a metal door into the facility grounds. A nurse shows you to the “dry” waiting area on the far side of a bright orange plastic fence, an area reserved for suspect cases not yet exhibiting the “wet” symptoms of diarrhoea and vomiting. A few metres away, in another fenced area, you see a chaotic scene: sick people lying on the floor, outnumbered workers in protective suits diligently working to clean diarrhoea and vomit from around them. A nurse in a protective suit approaches you and asks about your symptoms from a distance. She takes your temperature using an infrared device and asks you to wait. Six hours go by and the surveillance team arrives to take a blood sample. Then you wait. Your symptoms worsen the following day. You wait for days at the holding centre. You share bathrooms, eating utensils, and living quarters with up to 100 other sick people. On the third day, your result comes back: you have tested negative for Ebola.
Accurate and timely diagnosis of patients has been a key aspect of the response to the current Ebola epidemic in West Africa. Early isolation and care of patients suspected to be infected with Ebola is a critical public health measure required to prevent transmission of this deadly disease. The process for differentiating those who have Ebola from those who do not can pose a great danger to patients. Patients with unexplained fever that could be caused by Ebola virus are placed in quarantine wards until diagnostic tests can be completed. A special surveillance team is usually required to collect the sample and initiate contact tracing. During the height of the epidemic, laboratory and surveillance professionals were overwhelmed, which often led to long wait times and caused the patient a great deal of anxiety. Even after samples are collected, they must be transported to a laboratory with the capacity to perform the complex PCR-based tests required. Samples may have to be transported via numerous facilities, and then travel for hours by road or even air. Once samples arrive at the laboratory, they are stored until the test can be run. Collection delays, transportation difficulties, test capacity and result communication challenges often lead to average result turnaround times (in this case the time from sample collection to receipt of result) greater than six days. [1]
These delays present patients with an excruciating wait and, more importantly, put uninfected individuals at risk of being infected. [2] To reduce the delay between a patient’s arrival at the clinic and a confirmed diagnosis, researchers are working on rapid Ebola diagnostics at the point of care (POC). Two Ebola POC tests that have been developed in the last few months are an equipment-free serological test, which awaits prequalification by the World Health Organization (WHO) [3]; and a rapid integrated nucleic acid PCR test, which is undergoing clinical trials in Conakry, Guinea.
| POC update for Ebola |
| On 19 February 2015 the WHO approved the ReEBOV Antigen Rapid Test Kit eligible for WHO procurement. The test is able to detect Ebola protein, not nucleic acid, within 15 minutes. For more information visit: http://www.who.int/medicines/ebola-treatment/1st_antigen_RT_Ebola/en/ |
With Ebola, the stakes around rapid, accurate diagnosis are very high. But Ebola is not the only disease where a delay in diagnosis can cost lives. POC diagnostic tests are just as important in the battle against other acute and chronic diseases: working faster than conventional laboratory testing, and with less equipment, they can extend healthcare access into the community and reduce the number of patients lost to follow-up, or the number of treatments initiated too late. For many high-burden infectious diseases such as HIV, TB, malaria and others, earlier diagnosis and treatment can also mean the difference between life and death. “The advantage of having a POC test is that you don’t have to move patients, you can test them closer to community,” says Dr. Amadou Sall, Scientific Director of the Institut Pasteur of Dakar, Senegal. This accessibility can expand the reach of healthcare beyond what a conventional laboratory could do on its own.
As to exactly what “point-of-care” means, definitions differ. The WHO developed the ASSURED criteria of Affordability, Sensitivity, Specificity, User-friendliness, Rapid results, Equipment-free and Delivered to patients to describe the ideal POC diagnostic, which would bring the test to the patient in an expedient and timely manner. [4] This vision resembles a pregnancy test: a sensitive, accurate and specific test that requires little technical training to administer or interpret. In practice, very few POC diagnostics meet all of the ASSURED criteria. No one would call the nucleic acid Ebola POC test fully “equipment free”, since it requires a laboratory in a suitcase. Likewise, although the scale-up of GeneXpert MTB/RIF testing has revolutionised the availability of rapid, accurate diagnosis of tuberculosis (TB) and drug-resistant TB, the platform is designed for use in a laboratory and takes several hours. [5] Although these and other diagnostics marketed as POC tests cannot be carried out literally at the patient’s bedside or in the examining room, they improve on earlier methods by reducing the time and infrastructure required to deliver a diagnosis. However, some POC diagnostics, including HIV and CD4 rapid tests, are readily available and highly transportable.

“I think we’re at a point where the technologies we need are here, but we really need to accelerate their uptake,” says Jonathan Lehe, who manages the Point-of-Care Diagnostics programme at the Clinton Health Access Initiative. At this stage, experts agree that increasing access to existing technologies is a high priority for global health.
The question, then, is what currently keeps the tests that exist from being used? Among the major barriers to POC diagnostic access are the regulatory processes, pricing and funding, implementation challenges, and quality assurance, which will be further explored in this article.
Accuracy and affordability: A balancing act
In 2013, 58 million people were tested using HIV rapid tests; studies show that in various settings the performance of HIV rapid tests can be variable, [6] resulting in misclassification of false positives and false negatives [7] Although this is startling, it has to be considered against the number of correct diagnoses that would not have been made, or would not have reached patients, with the more expensive and complex gold standard of ELISA (enzyme-linked immunosorbent assay) serological testing followed by confirmatory Western blot. Ministries of Health (MOHs), non-governmental organisations (NGOs) and diagnostic experts around the world continue to debate whether expanding access is enough to justify sacrificing some measure of accuracy. According to one school of thought, if a test increases the number of diagnoses that can be made and reduces patient loss to follow-up, a higher false negative rate should be acceptable—the total number of correct diagnoses is still increased. However, another opinion holds that quality is paramount, keeping in mind that if costs are cut, then simpler and cheaper tests such as HIV rapid tests will not have the same accuracy as the gold standard.
The responsibility for setting accuracy parameters within a country or smaller markets within the country falls to each country’s MOH. “I think it’s appropriate for the WHO to set guidance on this sort of question, but in the end I think it should be up to the MOHs that are setting policies for the healthcare of their people,” says Lehe. As MOHs strike the balance between access and affordability, they must consider the consequences of each inaccurate outcome. For infectious diseases such as Ebola and HIV, the risks of an inaccurate test result are very high for the individual, for the health of others susceptible, and for the country’s healthcare budget. The dangers of a false negative on, for example, a pregnancy test are much milder. In either case, knowing the true accuracy of a test is important in making an informed regulatory decision about whether and where to allow it to be sold. Therefore, the first step in choosing to license, procure or implement a POC diagnostic is independent confirmation that manufacturers’ claims about false positive and false negative rates are accurate.
Product evaluation: The regulatory process
“To make a test available to people, one has to go through the dossier [process]: show that performance is good, that it meets manufacturing criteria, and that it meets the country’s rules for in vitro diagnostics,” says Dr. Sall. These tests are

initially carried out by the manufacturer, but must be verified by third parties including international bodies and national governments. Although these steps are crucial to protecting the health of the population from fraudulent or faulty diagnostic tests, analysts also point to regulation as a barrier to access of POC tests.
International health organisations such as the WHO and US Centers for Disease Control and Prevention (CDC) carry out substantial quality assurance work for POC diagnostics. The WHO prequalification team for diagnostics, for example, follows a workflow that begins with a thorough review of product dossiers, examining the product design and manufacturing quality management systems on paper. Dossier review is followed by a site visit to make sure the product’s manufacture matches its quality claims, and laboratory testing to confirm manufacturers’ claims about accuracy. If a diagnostic passes these tests, it can be prequalified for procurement by the United Nations (UN). Because the standards for prequalification are quite stringent, many national MOHs use prequalification or other assessment by organisations such as the United States Food and Drug Administration (USFDA) or the Conformité Européenne (CE) as a precondition for considering a diagnostic. The WHO prequalification branch publishes information including a list of prequalified in vitro diagnostics (IVDs) as well as a guideline for risk-based assessment of IVDs. [8]
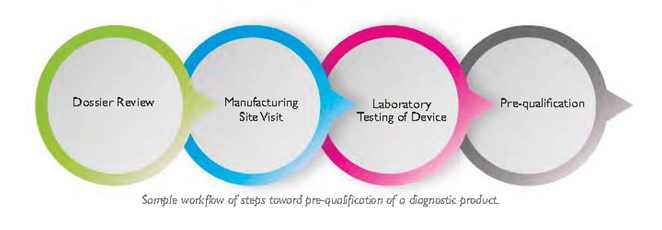 Technical assessment by an international agency is an important step toward introducing a diagnostic to a national market, but each country’s MOH and subordinate regulatory agencies usually have further regulatory requirements to fulfil. National standards may be different than the WHO standard; moreover, the conditions under which a test is used within the country may be different. For example, a recent field validation study of rapid HIV tests in a clinical setting in South Africa found that identical rapid diagnostic tests performed with lower sensitivity in the clinic than when they had been tested in the laboratory, perhaps because of training differences, environmental factors, or the use of serum in the laboratory and whole blood in the clinic. [9] In-country assessment can assure that a test will work reliably in the context where it is to be used.
Technical assessment by an international agency is an important step toward introducing a diagnostic to a national market, but each country’s MOH and subordinate regulatory agencies usually have further regulatory requirements to fulfil. National standards may be different than the WHO standard; moreover, the conditions under which a test is used within the country may be different. For example, a recent field validation study of rapid HIV tests in a clinical setting in South Africa found that identical rapid diagnostic tests performed with lower sensitivity in the clinic than when they had been tested in the laboratory, perhaps because of training differences, environmental factors, or the use of serum in the laboratory and whole blood in the clinic. [9] In-country assessment can assure that a test will work reliably in the context where it is to be used.
Although national-level testing is important for weeding out low-quality diagnostics, it can also be a barrier to putting effective tests onto the market. Redundant evaluations in many countries can take years and can become very expensive. Meanwhile, companies that produce diagnostics must navigate redundant approval processes in many small to medium markets around the world. Recognising that the regulatory process can become a barrier to the introduction of good and bad products into the market, many groups have made it a goal to hasten the evaluation of diagnostics, without compromising quality. Various actors, notably the Pan-African Harmonization Working Party (PAHWP) on Medical Devices and Diagnostics, are working to harmonise regional regulatory standards, with short-term goals that include a shared Registration File (the form that companies submit in order to apply for permission to market diagnostics); collaborative clinical studies for regulatory approval; regional post-market surveillance; and clearer standards for risk classification.
Another important goal for international bodies is to develop clear guidelines that help MOHs choose between available approved tests. As outlined in the previous section, choosing the appropriate diagnostic test can involve ethical as well as economic and technical considerations. Moreover, some markets, especially the HIV rapid test market, are crowded with very similar rapid diagnostic test products, presenting a challenge to individuals trying to choose the appropriate test for a whole country. Disease-specific diagnostic landscape reports compiled by international groups can be a good guide in the short term. [10] These reports incorporate performance, cost, operational characteristics such as throughput and shelf life, and can help MOHs to streamline their decisions about approving and purchasing diagnostics.
Price and funding
After a POC diagnostic passes the regulatory evaluations, it still must be purchased in order to benefit patients. In order to be purchased, it has to be both affordable and cost-effective. The per-test price of a POC diagnostic includes fixed production costs and the cost per unit produced; research and development costs including market assessment and biomarker assessment are also reflected in the price per test. These costs can run from USD $2 million to over $10 million and represent an investment of up to ten years, and are built into the cost of POC tests. [11] Per test, this can add up to a hefty fee, especially for systems such as the GeneXpert MDR/RIF test for TB (or various CD4 systems), which include both consumable reagents and expensive equipment.
A good potential pricing solution is for companies to set prices transparently, with a plan to reduce the price per test as sales volume increases. “If companies are afraid that volumes won’t materialise, so they set the price too high, it will be a self-fulfilling prophecy. If countries see an entry price that is too high, they may assume that this will be the price forever, and they may decide they can’t afford to introduce the product at all,” says Lehe. With a transparent, tiered pricing structure, scaling up use of a POC test can evolve from prohibitively expensive to palatable and cost-effective. Non-governmental organisations (NGOs) such as the Clinton Health Access Initiative mediate between producers and end users of diagnostics to improve access by sharing information more openly between the two sides. Meanwhile, countries can contribute to this effort by building test demand, sharing scale-up plans and forecasts, and consolidating testing carried out by many providers within their borders in order to secure bulk discounts.
Comparing the cost-effectiveness of POC and conventional diagnostics can help MOHs decide which of many options to implement. However, “just because something is cost effective doesn’t mean it’s affordable,” says Lehe. “We did a lot of work to demonstrate that POC CD4 is highly cost effective, but it still requires incremental investment, and if countries don’t have the money it won’t happen.” Supplemental funding for POC tests and other healthcare products often comes from global donors, for example Global Fund grants. While they have greatly improved access, these grants take time to be approved and delivered. Though they are well suited to meet entrenched health needs such as HIV and TB, the pace is poorly suited to respond to an outbreak situation or a ground-breaking new product. As a possible solution to minimise the disconnect between the need for and the availability of funds, a ministerial panel at ASLM2014 proposed a global “Diagnostics Access Fund.” Lehe describes this as funds “earmarked specifically for diagnostics, but flexible enough to meet changing needs.” [12]
When funding dictates that only a limited number of tests are available, targeting POC diagnostics to specific, high-risk populations can optimise the return on each test. For example, a rapid syphilis diagnostic is extremely cost-effective, in terms of preventing negative long-term health outcomes. Subsequent treatment can prevent the devastating long-term effects of congenital syphilis. If resources are limited, guidelines may suggest reserving the tests only for pregnant women, to obtain the greatest possible positive impact from each test, even though health benefits are available for any patient who can be cured of syphilis. [13]
When funds are available, it is often most cost-effective to mix high-volume laboratory tests with POC testing. [14] Cost per test on both existing laboratory diagnostic equipment and POC devices varies depending on the utilisation rate, with the highest cost savings achieved at full capacity. [15] One benefit of many POC tests which are equipment-free is that even with very low test volumes, the cost per test is fixed ahead of time, rendering testing cost-effective even at remote sites with low volume. On the other hand, many POC tests do require simple equipment, which may not be cost effective at very low volumes. At the same time, countries justifiably wish to maximise their return on existing investments in laboratory infrastructure. Developing a balance between diagnostic strategies depends on each country’s demographics, budget, and existing laboratory infrastructure.
Deploying POC diagnostics: Local buy-in
 “You can have the best technology, but if the community does not come to use it, it is useless,” comments Dr. Sall. Implementation of any new diagnostic test on a country-level scale is the result of many earlier decisions taken by its developers and manufacturers, international regulatory bodies, and government MOHs. However, the decision to deploy a test comes down to an individual clinician or community health worker. Without buy-in at the level of individual clinics, diagnostic tests chosen and purchased with great effort may sit unused in boxes.
“You can have the best technology, but if the community does not come to use it, it is useless,” comments Dr. Sall. Implementation of any new diagnostic test on a country-level scale is the result of many earlier decisions taken by its developers and manufacturers, international regulatory bodies, and government MOHs. However, the decision to deploy a test comes down to an individual clinician or community health worker. Without buy-in at the level of individual clinics, diagnostic tests chosen and purchased with great effort may sit unused in boxes.
A robust implementation strategy is required to ensure successful roll-out of new POC diagnostics and must include a plan for training the people who will administer the tests. This strategy may necessitate clinic workflow changes to improve testing efficiency. To reduce the burden an extra diagnostic puts on already-overworked healthcare workers, and to extend the reach of POC diagnostics, many countries have workforces with less clinical or laboratory training who are specifically trained to administer POC tests. In Kenya, the National Public Health Laboratory Service runs a proficiency testing system for these workers, and has found that targeting testers rather than sites helps to ensure quality delivery of diagnoses. [16]
The closer POC diagnostic testing becomes to the patient, the harder it becomes to consolidate data so that national-level authorities can analyse health outcomes countrywide. Thus, greater connectivity between central and peripheral facilities is required. Automated data transmission of test results from central laboratories to clinic sites reduces turnaround time for diagnostic tests performed away from the POC, and has been found to reduce human transcription errors in data reporting. [17] Some companies have taken on the challenge of enhancing connectivity to encourage uptake of their diagnostics; for example, POC products that can be connected to a central database by SIM. Lehe suggests that companies can provide greater technical support, including training and maintenance contracts for their highly specialised equipment, to reduce the logistical burden of delivering POC tests.
Procurement, post-market analysis and continuous quality monitoring
A new diagnostic test must be thoroughly validated prior to use in country. Additionally, standard procedures must be applied to ensure that products are selected, validated and purchased in a transparent manner. Once a product is selected and procured, post-market surveillance is required to ensure appropriate that diagnostic quality is maintained at the same level it had to reach in order to be introduced to the market. Continual quality monitoring is performed at the international level. For example, WHO periodically inspects manufacturing facilities of prequalified products. If a site fails to rectify quality concerns, the result is a Notice of Concern, a red flag that can affect the reputation of that product. WHO provides an online feedback form for complaints and product alerts, so that problems revealed by post-marketing surveillance efforts can be shared between nations.
Post-market surveillance is intended to provide early warning of product quality issues, but is not designed to serve as a quality assurance program for the users. Therefore, quality assurance programmes at sites that administer tests must be in place to ensure that the quality of diagnostic delivery matches its quality of manufacture. Quality assurance is a costly but critical process. During an ASLM2014 symposium, “Advances in Implementing Quality Assured Point-of-Care Testing”, speakers discussed the feasibility of dedicating one percent of programme costs to quality assurance by factoring these assessments into the cost per test. Countries can then respond to subpar diagnostics by removing them from the market to prevent harm to their citizens, or respond to sub-par service delivery by improving operator performance through re-training or site mentorship.
Changing diagnostic landscapes and ways forward
The landscape for diagnostics and especially POC is continually changing. In 2010, WHO updated its recommendations for monitoring treatment effectiveness in HIV patients on antiretroviral therapy (ART). Until that year, the international recommendation was combined clinical monitoring and CD4 count; however, new research showed monitoring viral load to be an earlier and more sensitive way to identify treatment failure. [18] Although the WHO still recommends CD4 testing when a patient is first diagnosed, in order to stage patients and start them on ART when appropriate, routine CD4 monitoring is being phased out in many places in favour of annual viral load monitoring.
Many in the laboratory medicine field recall the transition from CD4 to viral load monitoring with frustration. Although based on evidence and aimed at improving patient care and stemming the rise of drug resistance, the new guidelines exacted a cost in training time, equipment and supplies. Some countries changed their policies to phase out routine CD4 monitoring before viral load monitoring was rolled out to most patients, while viral load scale-up remains unaffordable in other countries. International funders also changed their purchasing protocols and funding priorities. The change presented a setback for decentralised ART monitoring due to the fact that although some CD4 POC technologies exist, there is as yet no POC test for viral load.
What should a country do when infrastructure it has invested a great deal to implement ceases to be the gold standard? ASLM, in partnership with WHO, the Joint United Nations Programme on HIV/AIDS (UNAIDS), and representatives from 20 MOHs, developed a set of recommendations to help countries navigate the transition to viral load testing. These include viral load testing on dried blood spots, which can be transported more cheaply. ASLM also recommends using existing capacity in early infant diagnosis laboratories to perform treatment monitoring, a guideline that underscores the importance of flexibility in both human resources and diagnostic strategies. [19]
As new technologies emerge, what is most cost-effective, most affordable, and most accurate will continue to change. Funding, regulation and implementation must remain flexible to these changing circumstances. In her keynote address at ASLM2014, Dr. Rosanna Peeling of the London School of Hygiene and Tropical Medicine (LSHTM) noted that in an increasingly connected world, roles for the medical laboratory have expanded to encompass surveillance for outbreak situations and resistance monitoring. Although the central laboratory will remain critical to the diagnosis of some conditions, as many routine diagnostic tests are outsourced from the laboratory and closer to the point-of-care, scientists and technicians may take on new roles. By participating in validation and post-market surveillance of POC diagnostics, laboratory professionals can help build a vibrant diagnostic network and make quality diagnostics available to the patients who stand to benefit most.
By: Laurel Oldach (Editorial Team); Contributors: Amadou Sall, PhD (Institut Pasteur, Dakar, Senegal), Jonathan Lehe, BA (Clinton Health Access Initiative), and Paula Fernandes, MBA, PhD (Editorial Team); Editors: Rachel Crane (Editorial Team) and Michele Merkel, MS (Editorial Team)
Originally published in the February 2015 issue of Lab Culture newsletter.
[1] WHO call for diagnostics. “Urgently needed: rapid, sensitive safe and simple Ebola tests.” 7 November 2014. Retrieved from: http://www.who.int/medicines/ebola-treatment/emp_ebola_diagnostics/en/
[2] Oldach, L (13 February 2015). Personal communication with Paula Fernandes.
[3] Chembio Update on DPP® Ebola and DPP® Febrile Illness Assays. Press release. 12 January 2015. Retrieved from: http://chembio.com/news-and-events/press-releases/
[4] Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. WHO Bulletin. Volume 90, Number 12, 2012 December, 914-920. Retrieved from: http://www.who.int/bulletin/volumes/90/12/BLT-12-102780-table-T1.html
[5] Tuberculosis diagnostics technology and market landscape (3rd Edition).UNITAID Secretariat, 2014. Retrieved from: http://unitaid.org/images/marketdynamics/publications/UNITAID_TB_Diagnostics_Landscape_3rd-edition.pdf
[6] Kagulire et al. Field evaluation of five rapid diagnostic tests for screening of HIV-1 infections in rural Rakai, Uganda. 2011 Jun, 22(6):308-309
[7] WHO reminds national programmes to retest all newly diagnosed people with HIV. 22 October 2014. Retrieved from: http://www.who.int/hiv/pub/vct/retest-newly-diagnosed-plhiv-full/en/point-of-care
[8] WHO. “A risk based approach for the assessment of in vitro diagnostics (IVDs).” 2014.
[9] Black, Mollendorf, Moyes, Scott, Puren and Stevens. “Poor sensitivity of field rapid HIV testing: implications for mother-to-child transmission programme.” BJOG 2009; 116:1805-8.
[10] Landscape and Technical reports. (n.d.). Retrieved February 19, 2015, from http://www.unitaid.eu/en/resources/publications/technical-reports
[11] Peeling R. and Mabey D. “Point-of-care tests for diagnosing infections in the developing world.” Clin Microbiol 16(8):2010.
[12] Ministerial panel at ASLM2014.
[13] “The Rapid Syphilis Test Toolkit: a guide to planning, management and implementation.” Accessible via idc-dx.org.
[14] UNITAID Secretariat. “HIV/AIDS Diagnostics Technology Landscape” 4th edition 2014.
[15] Farouk Umaru. “Cost effective mix of POC and conventional instrument deployment in Zambia.” ASLM2014 presentation, Oral Session “Return on investment in laboratory”
[16] Sophie Mwanyumba. “Rapid HIV testing, going beyond numbers in the era of task shifting to non laboratory personnel while maintaining quality through individual-based proficiency test monitoring: the Kenyan successful experience.” ASLM2014, Oral session 4.3, “HIV Proficiency Testing.”
[17] Edwin Ochieng. “Rift Valley Provincial General Hospital in Kenya goes paperless: achieving 100% automation of lab data in developing countries.” ASLM2014, oral session 4.4, “Sustainable Lab Information Systems.”
[18] WHO. “Consolidated ARV guidelines, June 2013.”
[19] “Viral Load Testing Consultation Meeting, Full Report” accessible at aslm.org/resource-centre/hiv-viral-load-testing

