Resource Centre
ASLM’s Resource Center is designed to help you keep up with the latest tool kits, regulations, guides and other information published by ASLM, partners, and global health regulatory bodies. Use the filters above to locate the information you need based on the ASLM project, resource type, or topic of interest. To find a specific resource, enter the resource name in the search bar. You may also use the search bar to enter key words related to the resources of your interest.
On 21 Mar 2024, ASLM, in collaboration with FIND, convened a LabCoP DNO Sub-CoP session focusing on Cote d Ivoire’s experience in the implementing diagnostic network optimization (DNO). The session examined how a pilot DNO exercise was designed, highlighting key findings and recommendations to improve access to diagnostic services for HIV, tuberculosis, and human papilloma… Read More
The March LabCoP ECHO Session focused on transforming pediatric HIV care through ensuring equitable access with the introduction of Xpert HIV-1 Qual XC. Early initiation of antiretroviral therapy (ART) in HIV infected infants improves overall care outcomes, yet coverage of critical interventions including access to timely diagnostic services in this population remains low. There is… Read More
The February LabCoP ECHO Session focused on low-level viremia during anti-retroviral therapy. Progress in research on HIV/AIDS, the implementation of interventions to support prevention, case identification and availing lifesaving antiretroviral treatment (ART) have been generally successful. Despite these strides, up to 39 Million people globally were living with HIV and about 630,000 died from AIDS-related… Read More
The Global Strategy on Human Resources for Health, launched at the 69th World Health Assembly in 2016, aims at having universal availability, accessibility, acceptability, coverage and quality of health workforce that supports effective health service delivery. In this session, we examine what the laboratory workforce of the 21st century should look like, including having more… Read More
LabCoP’s Waste Management ECHO session, held on 7 Dec 2023, focused on sustainable healthcare waste management. Having a comprehensive waste management system backed by competent policies/guidelines, competent staff, appropriate technologies chosen, deployed, and properly maintained is an obligation of every institution. Janita Hoffman, the Medical Sales Representative for Medi-Clave (Pty) Ltd, South Africa, presented an… Read More
The October 2023 LabCoP Diagnostic Network Optimization (DNO) Sub-CoP EHO Session was help in conjunction with the Kenya Ministry of Health and partners on integrated DNO. The session provided an overview of the integrated DNO conducted in Kenya to improve access to testing for tuberculosis (TB), HIV and human papillomavirus (HPV). Presentations were made by… Read More
On 19 October 2023, ASLM’s LabCoP convened an Extended ECHO session focussing on the Global Fund Strategy 2023-2028, and the priorities and updates for Grant Cycle 7. In the session, Juliet Bryant, Medical Laboratory Specialist at the Global Fund, shares information about key laboratory systems strengthening investment support, updates and perspectives for GC7. Please follow… Read More
The August LabCoP ECHO session, was jointly organized by ASLM and Global Laboratory Initiative (GLI), one of the 7 working groups of the Stop TB Partnership. This session was convened to review the World Health Organization’s Standard on Universal Access to Rapid Tuberculosis Diagnostics. The WHO End TB Strategy calls for all notified TB cases… Read More
The July 2023 LabCoP ECHO session, jointly organized by ASLM and Cepheid, focused on point-of-care (POC) HIV viral load (VL) at delivery to facilitate early infant diagnosis (EID) and maternal adherence in Uganda. In this session, we discuss results of a randomized trial focussing on the use of POC testing for EID and VL to… Read More
LabCoP’s Waste Management session, held on 6 July 2023, in collaboration with Africa CDC and the UK Health Security Agency, focused on detection and surveillance of chemical hazards. This is the second of two sessions dedicated to chemical waste management. Lydia Izon, the Principal Public Health Scientist with Chemicals and Poisons Team, Radiation, Chemicals and… Read More
LabCoP’s Waste Management ECHO session, held on 6 June 2023, focused on general principles of chemical hazards and public health. Laboratory testing and other healthcare procedures involve the use of chemicals that have the potential to cause harm. Knowledge of required precautions or mitigation measures in the event of exposures are key in ensuring safe… Read More
On Thursday, 8 June 2023, African Society for Laboratory Medicine convened a session to walk through the historic WHA resolution on Strengthening diagnostics capacity. This resolution, the first of its kind, that addresses diagnostics was initiated by the Kingdom of Eswatini, and co-sponsored by all WHO AFRO region member states, Indonesia, Bangladesh, Germany, France and… Read More
The June 2023 LabCoP ECHO session focused on using key performance indicators (KPIs) to drive laboratory network performance management. Just like any product or service industry, effective, efficient and reliable national laboratory network management relies on a deliberate and structured efforts to continuously look for critical areas that can aid or diminish performance and continuously… Read More
The May 2023 LabCoP ECHO session jointly organised by the African Society for Laboratory Medicine and the Global Laboratory Initiative (GLI) featured the Tuberculosis Diagnostic Network Assessment (TB-DNA) tool. TB-DNA is a country-driven process that assesses the functionality of the national tuberculosis (TB) diagnostic network and system to determine its capacity to meet the country’s… Read More
On 4 May, ASLM’s LabCoP, in collaboration with EDCTP and TB-CAPT, convened an Extended ECHO session that focused on exploring advancements in the lipoarabinomannan (LAM) diagnostic development pipeline, approaches for clinical adoption in field settings, and strategies for quality assessment. The information in this session helps address the urgent need for TB tools and diagnostic… Read More
This LabCoP Diagnostic Network Optimisation (DNO) Sub-CoP ECHO session in co-convened with the Foundation for Innovative New Diagnostics (FIND), focuses on providing an overview of the DNO analysis in Zambia for TB/HIV testing integration, including objectives, methodology and key findings. It further explores how findings from the diagnostic network optimization analysis informed Zambia Ministry of… Read More
The March 2023 LabCoP ECHO session organised by ASLM and the Global Laboratory Initiative (GLI) focused on the GLI Planning and Budgeting Tool for tuberculosis and drug resistant TB testing, and the Stop TB Partnership Global Drug Facility (GDF) diagnostics product catalogue. These planning and budgeting tools are semi-automated, editable, Excel-based calculation tools for estimating… Read More
A second February 2023 LabCoP Monthly ECHO session is one of a two-part series dedicated to the new WHO recommended HIV test algorithm. In this first session, we focus on transitioning to a three-assay HIV testing strategy that requires three consecutive reactive tests to provide an HIV positive diagnosis and limit the risk of misdiagnosis… Read More
On 16 Feb 2023, ASLM’s LabCoP convened an Extended session about an ultra-sensitive rapid diagnostic test for malaria; a potential solution for accelerating malaria elimination and its role in surveillance. Presentations were made by Luis Gonzalez, Global Director, Medical and Scientific Affairs; Xavier Ding, Clinical Strategy Manager, Infectious Disease Emerging Markets; and Carlos Cardenas, Global… Read More
The February 2023 LabCoP ECHO session is part 2 of The Revised ISO15189:2022 Standard series. It takes a deep dive into changes needed at country and facilities levels to align to and meet the requirements of the revised standard. The focus is on how to systematically handle the transition for both accredited and non-accredited laboratories…. Read More
The Waste Management ECHO session held on 2 February 2023 launched the second phase of LabCoP’s Waste Management Sub-Community of Practice. This new phase focusses on building from awareness creation to action, rallying all stakeholders in identifying concrete ways to improve and increase adoption and implementation of safer, practical, and sustainable methods/technologies for the disposal… Read More
This LabCoP ECHO session is the first in a four-part series dedicated to the revised ISO 15189:2022 version. In this session, we reintroduce key concepts of laboratory quality management systems (QMS) and cover some of the changes in the revised ISO 15189:2022, compared to the withdrawn ISO 15189:2012 version. In this session hosted by Beatrice… Read More
LabCoP’s first Diagnostic Network Optimisation Sub-Community of Practice (DNO Sub-CoP) ECHO session was held in September 2022. The DNO Sub-CoP is a collaboration between ASLM and FIND, that aims to improve the uptake and quality of DNO processes in LabCoP-participating countries. This joint session was moderated by Dr Marguerite Massinga Loembe, Senior Laboratory Adviser and… Read More
LabCoP’s Aug ECHO session focussed on measuring the progress and value of integrated sample transport systems for clinical and public health benefits. Access to health care, including diagnostic services, is one of the dimensions of quality. With regard to diagnostics, services are staggered through the healthcare system network with simple procedures at lower facilities, and… Read More
In LabCoP’s September ECHO session, ASLM and partners discussed optimising the TB/HIV diagnostic cascade through the implementation of the Clinic-Laboratory Interface Continuous Quality Improvement (CLICQ!) program. Optimal cascade performance requires that clinical and laboratory teams’ interface to ensure service continuity from identification of presumptive cases, specimen collection, transport and diagnostic testing, all the way through… Read More
This AMR CoP ECHO session explores surgical site infections and related antibiotic resistance profiles occurring after Bellwether procedures in rural Liberia. In this session, we explore the Liberian experience on antibiotic resistance profiles from surgical site infections that occur after surgery, the main causes of healthcare-related infections in low- and middle-income countries, and their impact… Read More
LabCoP’s December 2022 ECHO session shares practical insights on integrated testing for HBV and Xpert HCV to align with the World Health Organization’s goal for the triple elimination of HIV, HBV and syphilis. Using a case from Nigeria, we discuss implementation experiences at the point-of-care sites, the existing tuberculosis (TB) and HIV testing infrastructure and… Read More
This LabCoP Diagnostic Network Optimisation (DNO) Sub-CoP ECHO session focuses on promoting data-driven investments for effective laboratory network optimisation. In this session, the Global Fund, provides an overview of the GF funding cycle, priorities for involvement of Lab Directorates in preparing funding request, funding for diagnostic network optimization and how to plan the DNO lifecycle… Read More
On 17 November 2022, ASLM convened this special ECHO session on critical enablers and barriers for local manufacturing of diagnostics among African member states. The continent is heavily reliant on imported diagnostics, and in times of crisis, the continent is at greater risk, as supply chains tend to satisfy local needs first. True and equitable… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we learn about how Tanzania has managed to implement an AMR surveillance system based on the WHONET software and implementing the WHO Point Prevalence Survey on Antibiotic Use in Hospitals, as part of their National Action Plan on AMR implementation. We also discuss how this surveillance… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss the worrying pattern of AMR observed in children under-5 years in one region in Ghana and its implication on AMR National Action Plan (NAP) implementation. We also explore strategies adopted by Uganda to support AMR surveillance and the role of country Antimicrobial Resistance Coordinating… Read More
In this Antimicrobial (AMR) Community of Practice webinar, we explore how integrated AMR interventions have been applied in a One Health approach to improve surveillance. We discuss a One Health approach to containing AMR and the key role governments play in the global effort. We further share the country-specific context of how Kenya’s government contribution… Read More
On 7 July 2022, ASLM’s LabCoP convened a session about the power of human papiloma virus (HPV) DNA testing in transforming cervical cancer screening in Africa: In this session, In this session, we highlight the value of screening with the HPV DNA test, and its clinical performance. We also share the Zambia country experience of… Read More
On 14 July 2022, ASLM’s LabCoP convened a webinar about the Rapid Test Continuous Quality Improvement (RT-CQI) initiative developed by PEPFAR and the US Centre for Disease Control and Prevention. In partnership with other stakeholders, it supports the strengthening of capacity and ongoing assessment of facility and individual tester competency in performing rapid tests. Given… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss the One Health approach to addressing antimicrobial resistance (AMR). This approach provides significant opportunities to explore broadly-existing microbial relationships between humans and animals. In this session, from the genomic point of view, the Institut Pasteur de Dakar (IPD), Senegal presents unique perspectives of AMR… Read More
On 23 June 2022, this Extended ECHO session was convened to discuss best practices and the potential of lay health workers in supporting health services delivery, especially diagnostics at decentralised health facilities, the first point of contact for patients seeking healthcare support. In this discussion, Mr Zibusiso Ndlovu, Epidemiologist and Laboratory Advisor, Medecins Sans Frontieres… Read More
On 7 June 2022, this special ECHO session was held to focus on the Monkeypox virus outbreak. In this session, led by Dr Jacqueline Weyer, Principal Medical Scientist, Centre for Emerging Zoonotic and Parasitic Diseases, National Institute for Communicable Diseases, National Health Laboratory Service, South Africa; and Dr Placide Mbala, Head of the Epidemiology Department… Read More
In this In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we share the experience of Kenya in the development of a national quality manual and strategic plan for the animal health sector, supporting the establishment on a national quality infrastructure that supports and mandates quality management systems (QMS) implementation and external quality assurance (EQA)… Read More
LabCoP’s June 2022 session featured the Zimbabwe experience implementing electronic result reporting, using the SMS platform, and the impact it is having on the timely utilization of laboratory results and general HIV clients management. Laboratory results are used in the diagnosis and monitoring of treatment response among individuals with acute and chronic conditions/diseases. It is… Read More
On 31 May 2022, ASLM, in collaboration with the Stop TB Partnership Global Laboratory Initiative (GLI), EDCTP, and TB-CAPT project, convened a session on guidance in selecting patient-cantered, setting-specific solutions for tuberculosis (TB) detection. This new WHO/ Global Laboratory Initiative manual recommends that all patients with presumptive TB receive an initial, molecular WHO-recommended diagnostic (mWRD)… Read More
In this AMR CoP session, we discuss laboratory workforce development challenges. In many countries in Africa, the numbers and skills of professionals capable of generating quality antimicrobial susceptibility test (AST) laboratory results, interpreting antimicrobial resistance (AMR) data or designing relevant and representative AMR surveillance protocols required for solid AMR surveillance systems are scarce. In addition,… Read More
On 14 April 2022 ASLM’s LabCoP convened an Extended ECHO session about addressing diagnostic gaps in the tuberculosis (TB) care cascade, which featured discussion about the MTB/XDR assays and considerations for their implementation. Presentations were made by Dr. Kogieleum Naidoo, Head of the HIV and Tuberculosis Treatment Program at the Centre for AIDS Program of… Read More
On 7 April, ASLM’s LabCoP convened an Extended ECHO session about the role of diagnostic network optimization to achieve 95-90-0 for mycobacterium tuberculosis (MTB) disease elimination while scaling up testing. This webinar provided perspectives from Roche Diagnostics on the role of network optimisation to achieve 95-90-0 for MTB disease elimination; the deployment of some of… Read More
On 31 March 2022, ASLM’s LabCoP convened an ECHO session on supporting the implementation of diagnostics and laboratory systems investments for COVID-19 in the African region. With funding from The Global Fund and through their Stellar Project, and in collaboration with Africa CDC, ASLM, Association of Public Health Laboratories (APHL), and Clinton Health Access Initiative… Read More
In this Antimicrobial (AMR) Community of Practice (CoP) webinar, we discuss our approach to establishing regional external quality assessment (EQA) providers and the achievements and lessons learnt from the implementation of a regional EQA programme for AMR. This AMR CoP and webinar are supported by the Fleming Fund’s EQA Grant for Africa (EQuAFRICA), together with… Read More
On 15 March 2022, ASLM’s LabCoP convened a session to review efforts to increase community access to COVID-19 antigen rapid diagnostic testing (Ag RDT) services, drawing experiences from Nigeria and South Africa. A significant portion of the continent’s SARS-CoV-2 testing needs remain unmet. One key to closing this gap is a well-coordinated scale up of… Read More
On 10 March 2022, ASLM’s LabCoP convened a session on external quality assessment (EQA) for SARS-CoV-2 and other emerging viruses. EQA is a vital tool that provides objective evidence on the credibility of reporting test results, and labs are required to use it to monitor their performance. Conditions for the implementation of EQA vary, depending… Read More
LabCoP’s March 2022 ECHO session focused on generating demand for HIV viral load (VL) testing through targeted communications campaigns. For people living with HIV and on antiretroviral treatment, taking a VL test is essential in monitoring if the treatment is working. The World Health Organization recommends at least one annual VL test. Yet, knowledge around… Read More
LabCoP’s February ECHO session introduced the HIV Laboratory Waste Cost Assessment Framework (WCAF), an MS Excel-based tool co-developed by the US CDC and Roche Diagnostics, under a public-private partnership to support accurate quantification of solid and liquid wastes generated in PEPFAR-supported countries, to allow easy communication about waste management costs. The session was premised on… Read More
On 10 Feb 2022, ASLM held this ECHO session to cover genomic surveillance for SARS-COV-2 in Africa by Africa CDC’s Africa Pathogen Genomics Initiative (Africa PGI). This initiative, a continental program launched in October 2020, aims to establish pathogen genomics and bioinformatics capacity in support of disease surveillance. In its first year, the initiative supported… Read More
On 27 Jan 2022, this extended ECHO session was convened to review the experiences from Tanzania’s first Tuberculosis Diagnostic Network Assessment. The aim of this assessment was to holistically understand the TB diagnostic network, identify key strengths and weaknesses and provide evidence-based recommendations to improve the network in Tanzania. The presentation was made by Samwel… Read More
On 8 December 2021 this special ECHO session was held to focus on the role of the Diagnostic Evidence Hub in accelerating uptake of diagnostic innovations. The Diagnostic Evidence Hub, launched in 2020 by the FASTER Project and hosted on the ASLM website, provides easy access to consolidated regulatory and performance data for test methods… Read More
On 4 November 2021, ASLM`s LabCoP monitoring and evaluation (M&E) sub-community of practice, in collaboration with the World Health Organization, convened a session focusing on monitoring country level viral load (VL) programs. Countries are putting in place robust systems to evaluate HIV VL programs to determine if set objectives, desired outcomes and impactful results are… Read More
On 28 October 2021 this Extended ECHO session focused on neglected tropical disease laboratories, their potential to act as effective reference labs with effective implementation of robust quality management systems (QMS) including external quality assessment (EQA) programs. Presentations were made by Dr Andreas Gilsdorf, Head, German Epidemic Preparedness Team SEEG), German Agency for International Cooperation… Read More
On 14 October 2021 this Extended ECHO session was convened to discuss the basics of latent tuberculosis infection (LTBI), the critical importance of screening for LTBI in addition to active TB case finding. Experts discussed gaps in the current TB care cascade, considered various options for LTBI screening and highlighted the benefits of the test-and-treat… Read More
On 16 September 2021, ASLM’s LabCoP hosted another session in our series on manufacturers of COVID-19 diagnostics products. This session featured Siemens Healthineers and Thermo Fisher Scientific. The session focussed on innovations in SARS-CoV-2 to handle detection and surveillance of the fast-emerging variants of concern (VOC) and variants of interest (VOI) that have implications on… Read More
On 9 September 2021, this COVID-19 ECHO session was convened to discuss the evolving role of diagnostics in the COVID-19 pandemic response. Testing, when used effectively, is one critical tool that supports control of any disease outbreak including the COVID-19 pandemic. Swift case identification and contact tracing inevitably prevent further disease transmission. Researchers have worked… Read More
On 7 September 2021, this COVID-19 ECHO session was convened by ASLM, in conjunction with Africa CDC, the Clinton Health Access Initiative, and Last Mile Health to provide an orientation and overview of the COVID-19 Antigen Rapid Diagnostic Testing (Ag RDT) training e-learning course. The course, hosted online through the ASLM Academy, allows free and… Read More
On 2 September 2021, this COVID-19 ECHO session was convened to share details on the COVIOS antigen rapid diagnostic test (Ag RDT) technology, status of regulatory approval, requirements/consumables to operate the test, and how Ag RDTs can be deployed to support testing programmes for COVID-19 epidemic control. COVIOS, developed by the Global Access Diagnostics, a… Read More
LabCoP’s August 2021 ECHO session focused on sharing progress in HIV viral load (VL) monitoring among patients on antiretroviral therapy (ART) from eight sub-Saharan Africa countries between 2013 and 2018. Monitoring of VL suppression at individual and population level is necessary to ensure achievement of global epidemic control. Despite significant challenges that hinder uptake in… Read More
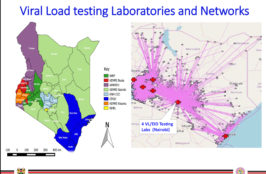
LabCoP’s July 2021 ECHO session focused on unlocking the potential of tiered laboratory networks through laboratory capacity mapping. Mapping the capacity of a national laboratory network is a powerful tool that supports visualizing what the testing capacity is, their location, and the population served. In this session, Dr Pascale Ondoa, Director of Science and New… Read More
On 15 July 2021 this Extended ECHO session was convened to discuss the WHO`s End TB Strategy that requires universal drug susceptibility testing and treatment of all tuberculosis case. Fewer than desired drug-resistant tuberculosis cases receive appropriate treatment due to either limited access to or sophisticated laboratory infrastructure needs. This webinar provided details on the… Read More
On 8 July 2021, this COVID-19 ECHO session was convened to discuss the perspective of Civil Society Organizations (CSOs) in leveraging on existing HIV community engagement to respond to COVID-19 challenges and opportunities. In this session, two CSOs, The National Forum for People living with HIV&AIDS Networks in Uganda (NAFOPHANO) and The Zimbabwe National Network… Read More
On 30 June 2021 this Extended ECHO session was convened to discuss the implementation of the Lateral-Flow Urine Lipoarabinomannan (LF-LAM) assay for the detection of active tuberculosis (TB) in people living with HIV. The session provided an overview of current World Health Organisation (WHO) recommendations, practical considerations to increase uptake, LF-LAM inclusion within the package… Read More
LabCoP’s June 2021 ECHO session focussed on generating demand for HIV viral load (VL) testing through targeted communications campaigns. WHO recommends at least one annual VL test for each person on Highly Active Antiretroviral Therapy (HAART) as this is essential in monitoring if treatment is working. Generally, knowledge around the importance of VL tests as… Read More
In June the ASLM LabCoP monitoring and evaluation (M&E) Sub-CoP held an ECHO session on viral load (VL) testing data quality assessment. The presentation provided an overview of the 2020 WHO-UNAIDS-PEPFAR-Global Fund module for assessing and strengthening the quality of VL testing data within HIV programmes and patient monitoring systems. Kenya shared their country’s VL… Read More
On 12 May 2021, this Extended ECHO session was convened to discuss the Global Fund COVID-19 funding mechanism, an opportunity for countries to request funds to strengthen their health systems in response while mitigating the effects of COVID-19 on other essential health services including HIV, tuberculosis (TB) and malaria. An innovative tool was discussed, that… Read More
In May 2021 the ASLM LabCoP monitoring and evaluation (M&E) Sub-CoP held a session on data management, dashboards and connectivity solutions for HIV viral load (VL) and early infant diagnosis (EID). Presentations were made by Yonatan Gossaye Dessalegn, Senior Associate Software Developer on Clinton Health Access Initiative (CHAI) Global Laboratory Services Team; Kalechristos Abebe, IT… Read More
On 13 May 2021, this COVID-19 ECHO session was convened to discuss a series of case studies on country experiences regarding the design and implementation of digital tools to manage the COVID-19 pandemic. The series was compiled by the Foundation for Innovative New Diagnostics (FIND) in collaboration with ministries of health and government partners. The goal was to promote… Read More
On 29 April, 2021 this COVID-19 ECHO session was convened to discuss tuberculosis (TB) and the COVID-19 pandemic. This panel discussion session was moderated by Rinuka Garde, Senior Advisor at the Clinton Health Access Initiative, and included three renowned experts in the field of TB and general lung health, including: Lucica Ditu, Executive Director Stop… Read More
On 22 April 2021 ASLM’s LabCoP M&E Sub-community of practice held a south-to-south learning session during which the Uganda country team shared their experience in rolling out monitoring and evaluation (M&E) systems for viral load (VL) including virtual dashboards that allow for performance tracking at various administrative levels. The session covered key aspects of data… Read More
On 15 April, 2021, ASLM’s LabCoP hosted the 13th session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott Rapid Diagnostics and Beckman Coulter Inc. In this session, Dr Anna Ruzhanskaya, Scientific Marketing Manager, CISEE, Beckman Coulter and Dr Kuku Appiah, Director Medical and Scientific Affairs-Abbot, share with participants some new developments… Read More
On 8 April, 2021 this COVID-19 ECHO session was convened to discuss the Africa CDC Trusted Travel Platform and how to create a ‘Trusted Network’ of COVID-19 testing laboratories in Africa. To support the reopening of boarders and economies without risking disease spread, Africa CDC and partners are championing the concept of Trusted Travel and… Read More
On 7 April, 2021, ASLM’s LabCoP hosted the 12th session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Thermo Fischer Scientific. This session also focussed on mutation and variant surveillance of SARS-CoV-2. Without a robust, coordinated universal effort to identify and characterize emerging variants, societies risk suffering significant setbacks in health care… Read More
On 1 April 2021, ASLM`s LabCoP M&E Sub-CoP, in collaboration with U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV and Tuberculosis (DGHT) convened a session focusing on indicators for scale-up of viral load (VL) testing and program outcomes. Presentations were made by Nadia Solehdin, an epidemiologist, and Kat Sisler, an evaluation… Read More
On 25 March 2021, this Extended ECHO session was convened to discuss the role of laboratory information management systems (LIMS) to collect, process, and store human biospecimens. Dr Dominique Anderson, Researcher, and Team Lead for Bio Collection Informatics at South African National Bioinformatics Institute, University of the Western Cape; and Prof Alan Christoffels, Director &… Read More
On 17 March, 2021 this COVID-19 ECHO session was convened to discuss outcomes, successes and lessons from implementing emergency SARS-CoV-2 external quality assessment proficiency testing in Africa. The African Joint taskforce on Coronavirus (AFTCOR) guidance objective 2 under Laboratory diagnosis is to ensure quality-assured testing for COVID-19 diagnosis, and strengthening reference laboratories and laboratory networks… Read More
On 5th March 2021, ASLM hosted a special COVID-19 session that looked at COVID-19 genomics surveillance and how mutations can affect diagnostics and Polymerase Chain Reaction (PCR). Genomic surveillance looks at the different sets of primers that are available and check the mutation into the primary targeted or binding sites. Mutations accumulate within the population… Read More
On 2 March 2021 this COVID-19 ECHO session was convened to discuss the African experience in expanding COVID-19 testing using Antigen Rapid Diagnostic Tests (Ag RDT). It has been over one year since COVID-19 was declared a pandemic by WHO. There is still much concern that COVID-19 infections are increasing in some countries after several… Read More
On 18 February 2021, the LabCoP M&E Sub-Community of Practice held its second session that unpacked the fundamentals of monitoring and evaluation (M&E) and its importance in viral load (VL) monitoring. The use of VL data is essential for patient-level and program-level decision making. VL data is often unavailable, delayed, or underutilised, hindering monitoring interventions… Read More
On 11 February 2021, ASLM, in collaboration with the US Centers for Disease Control and Prevention’s International Laboratory Branch (CDC ILB) and partnership with Roche, hosted a webinar aimed at creating access to tools that will help laboratories improve their activities and services for viral load (VL) and early infant diagnosis (EID). The presentation provides… Read More
On 12 February 2021, this COVID-19 ECHO session was convened for Prof Tulio de Oliveira to discuss the work of the Network of Genomic Surveillance of South Africa (NGS-SA) and the need to build a genomic network in Africa. From the beginning of the COVID-19 pandemic, the NGS-SA started genetic sequencing early enough by building… Read More
On 5 February 2021, ASLM’s LabCoP hosted the first ECHO session of the Monitoring and Evaluation (M&E) sub-community of practice. M&E assesses the performance of projects and programs set up by organizations to improve current and future outputs. A robust M&E system is critical to assess the performance of testing services and for decision making…. Read More
LabCoP’s January 2021 ECHO session covered PEPFAR’s Country Operational Plan 2021 and the laboratory systems strengthening priorities of the year. Dr George Alemnji, Senior Technical Advisor for PEPFAR Laboratory Services (OGAC), Washington DC, presented during the session. Please follow the links posted here to view the recorded video session on ASLM’s YouTube channel, and download… Read More
On 2 February, 2021 this COVID-19 ECHO session was convened to discuss expanding COVID-19 testing at schools, borders and at the workplace, in an effort to reopen economies. As many countries over the world are experiencing the second wave of COVID-19, forcing some to go back to stringent lockdown measures, countries are grappling with how… Read More
On 29 January 2021, ASLM’s LabCoP hosted the 10th session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring BD (US) and DiaSorin (Italy). Laboratory diagnosis remains the cornerstone for the control of COVID 19. New diagnostic methods with higher sensitivity and specificity, as well as faster results, are necessary and are continuously… Read More
On 22 January, 2021 this COVID-19 ECHO session was convened to discuss testing strategies for COVID-19 antigen rapid diagnostic tests (Ag RDT) and Africa CDC’s testing guidelines and recommendations. The start of 2021 has seen new challenges arise in the COVID-19 pandemic, with rapidly growing spread and new virus variants. To urgently address this, the… Read More
In December LabCoP conducted the inaugural session of the new LabCoP Extended series to introduce the Score-TB package, an integrated set of assessment tools for supporting the implementation of a QMS in laboratories performing tuberculosis tests. The Score-TB package was launched by Foundation for Innovative New Diagnostics (FIND), in collaboration with the Global Laboratory Initiative… Read More
On 12 November 2020, this COVID-19 ECHO session was convened to discuss the launch of the SARS-CoV-2 antigen rapid test training package developed by ASLM and Africa CDC, in partnership with CHAI, AFENET, AMREF, and Last Mile Health. The training package, available to all African Union Member States, aims to support the scale-up of SARS-CoV-2… Read More
On 27 October 2020, this COVID-19 ECHO session was convened to discuss funding and procurement mechanisms for SARS-CoV-2 antigen rapid tests. As countries prepare to roll out SARS-CoV-2 antigen testing, understanding funding and procurement mechanisms will be critical in ensuring countries have access to adequate rapid tests. Several countries are planning to secure antigen tests… Read More
On 23 October, 2020 ASLM’s LabCoP hosted the ninth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring STANDARD Q COVID-19 antigen rapid diagnostic test (Ag RDT). A new global partnership by WHO, The Global Fund, UNITAID, Africa CDC, FIND and other partners ensures that low-priced, high-quality Ag RDTs are available internationally… Read More
On 21 October, 2020 ASLM’s LabCoP hosted the ninth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott’s Panbio Ag RDT, an in-vitro diagnostic test for the qualitative detection of SAR-CoV-2 antigen. In this session, Dr Kuku Appiah, Director, Medical & Scientific Affairs, Africa, Abbott Rapid Diagnostics discuss the Abbott Panbio… Read More
On 14 October, 2020 ASLM’s LabCoP dedicated a special session to digital solutions in support of health care providers, systems, and stakeholders, and how they have been applicable to the COVID-19 pandemic. It is increasingly clear that adopting digital technology and integrating it into Ministries of Health (MoH) policy is key for smooth coordination and… Read More
On 9 October 2020, this COVID-19 ECHO session and expert panel was convened to discuss what, why, and how the COVID-19 antigen rapid diagnostic tests (Ag RDTs) can be rolled out for optimal clinical and public health outcomes in Africa. Ag RDTs are fast and easy-to-deploy diagnostic methods that take 10 – 15 minutes to… Read More
On 5 November, 2020 this COVID-19 ECHO session was convened to discuss considerations of pooling specimens for testing by real-time RT-PCR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection, also known as COVID-19 diagnosis, is performed using a molecular technique referred to as real-time reverse transcriptase-polymerase chain reaction (RT-PCR). This has remained the method of… Read More
On 10 September 2020 LabCoP hosted a special ECHO session about establishing diagnostic connectivity for laboratory network data flow across conventional and point-of-care systems. More than before, use of clinical laboratory data to inform decision making has become crucial in both direct patient care and in local, regional and international healthcare systems programming. The emergence… Read More
LabCoP’s August ECHO session covered external quality assessment (EQA) proficiency testing coverage, results and sustainability from the perspective of a service provider. EQA provides a measure of performance of testing laboratories and helps identify deficiencies and opportunities for improvement across the entire testing cascade. In this session, we discuss integrating economic principles into EQA, to… Read More
On 18 September 2020, this COVID-19 ECHO session was convened to discuss antigen testing’s role in helping control the COVID-19 pandemic. Real time Polymerized Chain Reaction (rt-PCR) testing to detect SARS-CoV-2 has been the WHO recommended method to diagnose COVID-19. However, this method has not always been available at every tier of the laboratory network… Read More
On 17 September 2020, this COVID-19 ECHO session was convened to discuss self-collection of nasal specimens as a means to lowering the barrier to SARS-CoV-2 testing and access to care. Diagnostic testing for SARS-CoV-2 remains a challenge today, even months since the outbreak of the pandemic and with the emergence of several innovations. In the… Read More
On 13 August 2020, this COVID-19 ECHO session was convened to discuss optimization of diagnostic networks and their key role in responding to COVID-19. Optimised national diagnostic networks are key to increasing access to high-quality diagnostics. With the outbreak of the COVID-19 pandemic, some sub-Saharan African countries leveraged more than a decade of investments in… Read More
On 6 August 2020, this COVID-19 ECHO session was convened to discuss laboratory data management considerations for COVID-19 response. Long turnaround times (TAT) for laboratory test results and poor data quality are chronic problems for many testing services. Timely and accurate testing services for COVID-19 are essential for monitoring the spread of the pandemic and… Read More
On 15 July 2020, this COVID-19 ECHO session was convened to discuss the public health lab networks and SARS-CoV-2 testing challenges, strategies, and lessons learned from the USA. During emergencies like COVID-19 when laboratory testing services surge, there are risks of a health care system being overwhelmed. Well-structured strategies must be formulated to cope with… Read More
On 10 July 2020, ASLM’s LabCoP hosted the eighth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Roche Diagnostics International. Presentations were made by Dr Wim van der Helm, Head of Healthcare Development, Roche Diagnostics, Europe, Middle-East, Africa and Latin America, and Dr Matthias Strobl, Senior Clinical Science Leader, Infectious Diseases,… Read More
On 8 July 2020, ASLM’s LabCoP hosted the seventh session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring EUROIMMUN AG, and Hologic. The session covers: overview of available SARS-CoV-2 laboratory test kit(s) e.g., what genes are targeted; performance, run time and other technical considerations of SARS-CoV-2 molecular diagnostics; requirements/consumables not supplied with… Read More
LabCoP’s June ECHO session covered differentiated service delivery (DSD) for HIV care. DSD is a client-centered approach aimed at simplifying and adapting HIV services to different groups of people living with HIV, while reducing the burden on the health system. In recent months, the response to the COVID-19 pandemic has included adjustments to clinical care… Read More
On 18 June 2020, ASLM’s LabCoP hosted the sixth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring PerkinElmer and Pro-Med. Stephanie Wilbraham, Market Development Manager, PerkinElmer, and Neil Barker, Co-Founder, Pro-Med Diagnostics, South Africa presented about the overview of their available SARS-CoV-2 laboratory test kit(s), performance, run time & other technical… Read More
On 11 June 2020, ASLM’s LabCoP hosted the fifth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring KH Medical and Inqaba Biotec. Dr Aron Abera, Technical Support Manager, Inqaba Biotec, South Africa; Dr Julius Muhumuza, Technical Advisor, KH Medical Co., Ltd.; Mr Adam Hong, CEO, KH Medical Co., Ltd.; and Dr… Read More
On 2 June 2020, this COVID-19 ECHO session was convened to discuss establishing an integrated specimen referral system (SRS) for SARS-CoV-2 testing recommended to enable access to testing services across the entire health pyramid. Many countries struggled to operationalise this guidance and failed to create sustainable and efficient sample transportation systems. Due to various challenges,… Read More
On 28 May, 2020 ASLM’s LabCoP hosted the fourth session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott. Presentations were made by Dr Claudio Galli, Associate Director, Medical Scientific Affairs Global, Abbott Core Laboratory, and Dr Liane Bauer, Director, Professional Services EMEA, Abbott Core Laboratory. They covered topics including detection of… Read More
During severe shortages of personal protective equipment (PPE) due to COVID-19, one solution to consider is decontamination and extended use of medical masks and N95/FFP2 respirators for health workers at the frontline. On 26 May, 2020 ASLM’s LabCoP hosted a session that covered these considerations and additional recommendations on homemade masks that have protection equal… Read More
On 20 May 2020, this COVID-19 ECHO session was convened to discuss procurement and the supply chain for COVID-19 diagnostics. Dr Lara Vojnov, Diagnostics Advisor at the World Health Organization, Geneva, and Dr Yenew Kebede, Head of Laboratory Services at Africa CDC presented about the need to coordinate and collaborate test availability and access; current… Read More
LabCoP’s May ECHO session covered the key considerations for expanding COVID-19 capacity to the sub-national level and featured the Ethiopian experience. During this session, participants learnt about the need to scale up diagnostic capacity; governance and coordination issues essential for expansion of COVID-19 testing; leveraging on existing capacity to cope with the needs; strategies for… Read More
The May 2020 Waste Management training session focused on SARS-CoV-2 laboratory biosafety guidance and featured the presentation of David Bressler, Health Scientist and Microbiologist at the International Laboratory Branch of the U.S. Centers for Disease Control and Prevention. Nearly all countries are scaling up COVID-19 testing as one of the main strategies to eliminate the… Read More
On 14 May 2020, this COVID-19 ECHO session was convened to assist the African laboratory community in designing and implementing an effective COVID-19 quality assured testing program. Mr Patrick Mateta, Vice President, Global Health Partnerships, at Clinical and Laboratory Standards Institute (CLSI) presented about how to plan and prepare for COVID-19 testing, the selection and… Read More
On 8 May 2020, ASLM’s LabCoP hosted part 2 of an ECHO session about COVID-19 serology tests. Serological diagnostic testing has been proposed to be useful in combination with molecular testing, in confirming COVID-19 infection or exposure to the virus. Serology tests also have the potential to be delivered in rapid format and at the… Read More
On 30 April 202, ASLM’s LabCoP hosted the third session in a series about manufacturers of COVID-19 molecular diagnostic tools, featuring Abbott and Bio-Rad. Dr Danijela Lucic, Senior Scientific Affairs Manager, Global Infectious Disease, Abbott Molecular, and Dr Marcus Neusser, EMEA Product Manager, Gene Expression, Bio-Rad, Germany, and Mr Richie Petronis, Product Manager, Bio-Rad, USA,… Read More
On 29 April 2020, ASLM’s LabCoP hosted this ECHO session on strategies to implement fast turn-around laboratory testing for control of COVID-19, presented by Dr Trevor Peter, Senior Director, Diagnostic Services, Clinton Health Access Initiative. Nucleic acid testing is the WHO-recommended method for detecting SARS-CoV-2. The global scale-up of COVID-19 diagnostics will depend on use… Read More
On 24 April 2020, Professor Rosanna Peeling and colleagues from the International Diagnostics Centre at the London School of Hygiene and Tropical Medicine pulled together evidence for and against the original set of recommendations for use of serologic tests. Prof Peeling was joined by Dr Yenew Kebede from Africa CDC and Dr Amadou Sall from… Read More
LabCoP’s April ECHO session featured presentations about maintaining HIV and TB testing in the context of COVID-19 from Dr George Alemnji, Senior Technical Advisor for Laboratory Services at the State Department Office of Global AIDS Coordinator (SGAC) and Health Diplomacy, US CDC; Dr Lara Vojnov, Diagnostics Advisor, WHO; and Dr Dennis Falzon, Medical Officer, World… Read More
On 16 April 2020, ASLM’s LabCoP hosted the second session in a series about manufacturers of COVID-19 molecular diagnostic tools. Dr Davide Manissero, Chief Medical Officer of Infection and Immune Diagnostics at QIAGEN, and Mr Riegardt Johnson, Field Application Scientist at Thermo Fisher Scientific presented about their available COVID-19 laboratory test kit(s), their status regarding… Read More
On 14 April 2020, ASLM’s LabCoP hosted the first session in a series about manufacturers of COVID-19 molecular diagnostics tools, including BD and Cepheid. Dr Charles Cooper, Global Vice President, Medical Affairs, Integrated Diagnostic Solutions at BD, and Ms Rujeko Tsomondo, Regional Application Lead at Cepheid presented about their available COVID-19 laboratory test kit(s), their… Read More
On 8 April 2020, ASLM and LabCoP convened a special ECHO session on lessons the African continent can learn from the Korean experience in controlling COVID-19 Dr Seon Kui Lee, Director, Division of Risk Assessment & International Cooperation at the Korea Centers for Disease Control and Prevention (KCDC), shared an overview of the KCDC and… Read More
On 30 March 2020, ASLM and LabCoP convened a special ECHO session on the latest available technology to diagnose COVID-19, and recommendations for clinical care and surveillance. Dr Cassandra Kelly-Cirino, from the Foundation for Innovative New Diagnostics (FIND), and Prof Rosanna Peeling, from the London School of Hygiene & Tropical Medicine, gave an overview of… Read More
On 25 March 2020, ASLM and LabCoP convened an ECHO session on troubleshooting common challenges associated with establishing diagnostic testing for SARS-CoV-2 (the causative agent of COVID-19). Dr Jinal Bhiman, Director of the National Influenza Centre at the NICD, South Africa, presented practical solutions to address the most common challenges encountered by laboratory staff recently… Read More
The March 2020 waste management training session focused on Hologic’s Best Practices for Waste Handling. Todd Richmond of Hologic, shared his experiences, expertise and advice on the waste and contamination management of Hologic’s Panther. In collaboration with the African Society for Laboratory Medicine (ASLM) and the LabCoP community, the CDC International Laboratory Branch (ILB) offers… Read More
In this session, colleagues from Kenya shared findings from the assessment of waste handling practices in their viral load (VL) and Early infant diagnosis (EID) laboratories. They discussed contextual issues in regard to VL & EID laboratory testing capacity and networks, laboratory self-assessment using a VL & EID customized assessment checklist, findings/results, lessons learnt, challenges… Read More
The November 2019 Waste Management training session focused on the perspectives of Abbott Laboratories’ Best Practices for Waste Handling. Delfin Rubin, Global Product Manager of HIV Care for Infectious Disease Emerging Markets at Abbott Rapid Diagnostics, and Ami Soni, EHS Lead for Abbott Molecular describe the constituents of their HIV rapid point-of-care diagnostics, such as… Read More
In this session we focused on the issues of policy, legal and regulatory frameworks for waste management, using an example from South Africa. This session underscored the fact one of the common noted challenges in many settings was that there were and still are limited or no existing national policies or guidelines on dealing with… Read More
This Waste Management session covered updates to the checklist for assessments of viral load (VL) and early infant diagnosis (EID) testing laboratories and associated healthcare facilities. David Bressler, MS, CBSP (SM) NRM of the International Laboratory Branch, CDC, Division of Global HIV and TB presented to the audience about the checklist/tool and its ability to… Read More
In the May 2019 session, Dr Katrina Sleeman, Associate Service Fellow in the Viral Load/Early Infant Diagnosis Team at the CDC ILB introduced a draft of a viral load WM checklist tool that can be used as baseline audit of select country VL labs. The tool aims to assist in creating awareness of best practices… Read More
In collaboration with ASLM and the LabCoP community, the CDC International Laboratory Branch (ILB) presented an overview of the World Health Organization (WHO) Publication: ‘Safe Management of Wastes from Health-Care Activities’ during the second ECHO session dedicated to waste management. This document, developed by WHO in 2017, highlights the key aspects of safe healthcare waste… Read More
In collaboration with the African Society for Laboratory Medicine (ASLM) and the LabCoP community, the CDC International Laboratory Branch (ILB) presented the first session in a series of ECHO sessions dedicated to waste management. In this session we reviewed waste management practices at the country level using the responses to the November 2018 LabCoP Waste… Read More
This presentation by Dr. Ritu Shrivastava from the International Laboratory Branch of the US Centers for Disease Control and Prevention covers free online, didactic and in-person resources available for global system strengthening through three public-private partnerships (PPP). These partnerships between the President’s Emergency Plan For AIDS Relief (PEPFAR) and three private entities (Becton Dickinson and… Read More
In September 2018, Solange Baptiste and Helen Etya’ale from International Treatment Preparedness Coalition (ITPC) presented about HIV viral load testing (VLT) demand creation. These presentations contain helpful information that country teams can use to improve demand creation for their HIV VLT programs. This includes information about the significance of demand creation for VLT and the… Read More
This four-step overview of Laboratory African Regional Collaborative (LARC) was presented by Patricia Riley from the International Laboratory Branch of the US Centers for Disease Control and Prevention. LARC can be used by countries to address inefficiencies at the laboratory–clinical interface at clinic level, and achieve the 3rd ‘90’ of the UNAIDS 90-90-90 HIV targets…. Read More
Kenya’s presentation during the May LabCoP ECHO session included their experiences implementing the ‘Plan, Do, Study, Act’ (PDSA) cycle to prioritise and rapidly test ‘change ideas’ needed to facilitate the rapid identification of unsuppressed viral load (VL), followed by swift appropriate clinical action. Please follow the links below to watch the recorded session on ASLM’s… Read More