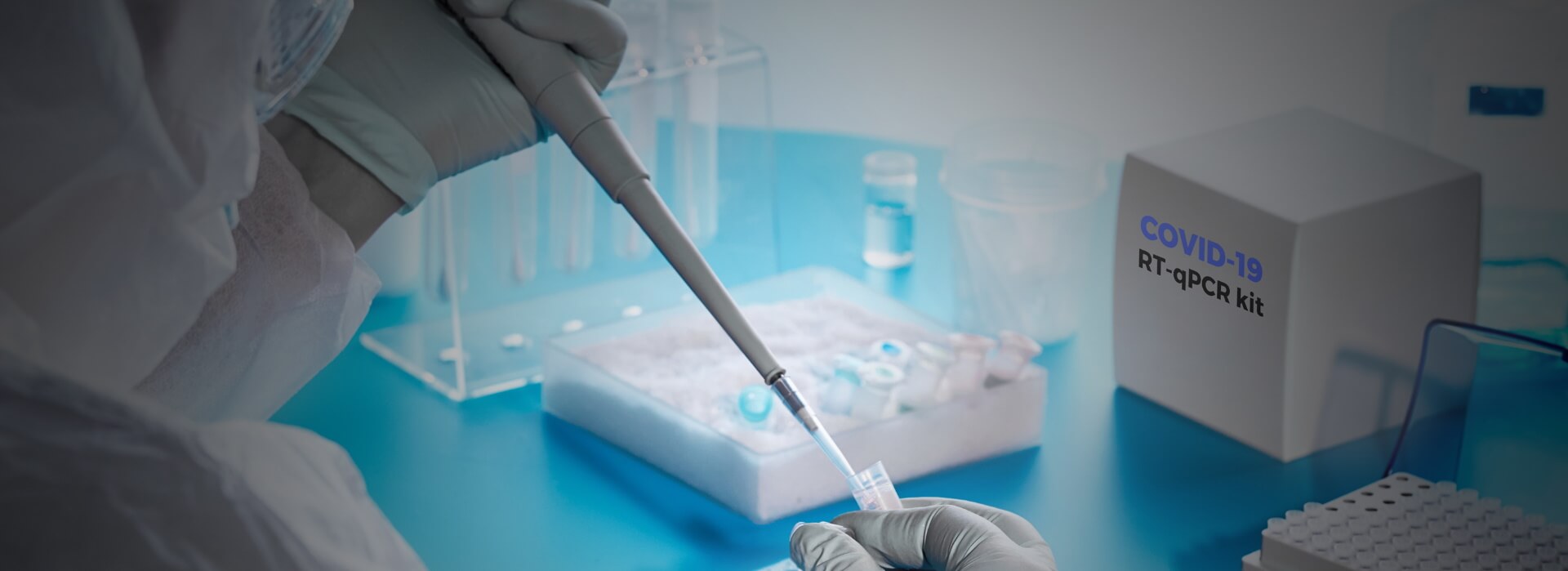
CoLTeP
What is CoLTeP?
It is critical to implement quality assurance and biosafety and biosecurity measures in all COVID-19 testing laboratories. To support this, ASLM, in collaboration with Africa CDC and other partners and Member States from Africa, has developed a COVID-19 Laboratory Testing Certification Program (CoLTeP). Through standardised processes, CoLTeP evaluates a facility’s readiness and continued suitability to conduct quality COVID-19 testing safely. Where deficiencies are identified, ASLM will assist in implementing corrective measurements and monitoring their effectiveness. The program will offer recognition of incremental implementation of stated requirements and award a 0–5-star rating of facility performance. Only facilities obtaining a 5-star rating (90%) of requirements will be authorized to perform COVID-19 testing and qualify for listing on the Africa Union Trusted Travel platform as an approved and Ministry of Health-recognized testing facility.
What is CoLTeP?
CoLTeP supports a variety of initiatives aimed at controlling the pandemic.

How Does It Work
To ensure sustainability as well rapid scalability, ASLM will use locally trained and certified assessors to conduct assessments. The designated focal person/office from the Ministry of Health is responsible for identifying potential testing facilities and collect required documentation to submit to ASLM Secretariat. The MoH shall consider the potential throughput of the applicant laboratory to ensure acceptable turnaround times will be achieved.
At minimum, the following documents shall be submitted:
- completed application form
- certificate of registration/authority to practice as a laboratory (if private laboratory).
- self assessment report conducted using the CoLTeP checklist
NOTE: Where a laboratory is re-applying (at least 3 months after last assessment), the application shall be accompanied by a completed laboratory action plan with documented evidence of effectives of implemented corrective actions.
Upon review, a letter of notification of enrolment is issued by ASLM Secretariat with communication of assessment logistics. At the completion of the assessment, audit reports are submitted to the ASLM Secretariat for approval and award of certificate valid for 12 months. The COVID-19 Testing Certification Program is comprised of the following stakeholders: Ministries of Health, applicant laboratories, COVID-19 Laboratory Testing Certification auditors and ASLM.
To ensure coordination, laboratories apply to Ministry of Health (MoH) requesting certification. MoH then prioritizes potential applicant testing facilities and submit approved applicants to ASLM Secretariat for enrolment.
Laboratory already with ISO Accreditation for their COVID-19 test do not need to apply for CoLTeP but will be automatically adopted into the African Union Trusted Travel Platform.
Assessment Process
• Designates a focal point responsible for coordination, information-sharing and implementation.
• Develops an implementation plan for COVID-19 Laboratory Testing Certification with prioritization of potential applicant laboratories.
• Allocates financial and human resources.
• Oversees the implementation of corrective actions outlined in audit reports.
• Coordination of certification program in-country.
• Receiving and screening applications, submitting to ASLM, receiving assessment reports and recommendations from ASLM
• Implement COVID-19 testing requirements.
• Submitting application to MoH
• Support the assessment process.
• Implement corrective measures for identified gaps.
• Developing certification program and assessment tools
• Training and certification of assessors.
• Reviewing applicants from MoH
• Deploying assessment teams
• Submitting assessment reports and recommendation to MOH
• Issuance of certificate of compliance
• Maintaining database of certified laboratories in collaboration with Africa CDC Trusted platfom
• Provide Technical Assistance in implementation and monitoring effectiveness of corrective actions